Physiology: The Force Behind Healthcare Simulation – A Guide for Techs, Part 6B: Hypovolemia Treatment & Tips for Sim
Learners are more likely to focus on desired learning objectives when they participate in life-like scenarios and receive information which is consistent with the healthcare situation being studied. This article, which is part 6B of a series entitled Physiology, The Force Behind Simulation – A Guide for Sim Techs, covers the physiological response, treatment and incorporation of hypovolemia into simulation. A previous article, Part 6A (linked below with the other series articles) discusses the physiological basics of Hypovolemia. The key to making realistic medical simulation scenarios is to understand how physiological functions change in response to various stimuli (stressors). Today’s article was guest authored by Kim Baily PhD, MSN, RN, CNE, previous Simulation Coordinator for Los Angeles Harbor College and Director of Nursing for El Camino College. Over the past 16 years Kim has developed and implemented several college simulation programs and previously chaired the Southern California Simulation Collaborative.
Responses to Hypovolemia
Cardiac output and blood pressure depend on circulating blood volume. As volume is lost, physiological mechanisms switch on to maintain blood pressure and ensure that tissues and organs are supplied with the oxygen they need. Clinicians assess signs and symptoms to determine the severity of blood loss and the required treatment.
Sponsored Content:
- The body’s response to the blood or fluid loss depends not only on the amount of blood lost but also how rapidly the blood was lost. When blood loss is rapid, compensatory mechanisms do not have time to respond quickly enough to maintain tissue perfusion.
- Initially arterial and cardiopulmonary baroreceptors are activated which is turn stimulate the sympathetic adrenergic nervous system. Heart rate and contractility increase and peripheral blood vessels constrict.
- Typically, a blood loss of <15% of total blood volume leads to small increases in heart rate and arterial blood pressure is maintained or may be elevated with a narrowed pulse pressure.
- Blood loss 15 to 40% – mean arterial and pulse pressures fall, and heart rate increases.
- When blood loss is severe, hemorrhagic shock develops. This is a life-threatening circulatory failure where the oxygen delivery is not enough to match tissue demand
- Greater than 40% blood loss is life threatening, severe hypotension, organ failure (MOF) and death may occur without adequate intervention.
- Other compensatory mechanisms, signs and symptoms.
- Sympathetic stimulation of the adrenal glands stimulates the release of catecholamines into the blood, which reinforce the effects of sympathetic activation on the heart and vasculature.
- Palpitations and diaphoresis (sweating) may occur.
- Orthostatic hypotension –
- Reduced organ blood flow leads to acidosis.
- Cells switch from aerobic to anaerobic metabolism leading to lactic acidosis. Changes in the blood lactate may be used to reflect the severity and course of shock and roughly predict outcome.
- Blood is diverted from organs to maintain blood flow to the heart and brain, more tissue ischemia occurs and worsening lactic acidosis.
- Extremities become pale, clammy and cold.
- A fall in capillary hydrostatic pressure occurs. This pressure normally drives fluid across the capillary endothelium, and into the interstitial space. When less fluid leaves the capillaries, and when the pressure falls sufficiently low as occurs following moderate-to-severe blood loss, net reabsorption of fluid can occur from the tissues back into the capillary plasma.
- Up to 1 liter/hour of fluid to be withdrawn from interstitial spaces back into the plasma.
- The patient may complain of air hunger and have rapid shallow breathing.
- The kidneys release renin leading to increased circulating levels of angiotensin II and aldosterone.
- Vascular constriction occurs
- Vasopressin is released
- Thirst mechanism is activated
- The kidneys increase reabsorption of sodium and water leading to increased blood volume.
- Urine output is decreased and may become darker and more concentrated.
- If the brain does not receive sufficient oxygen, the patient may become light-headed, confused, restless, fatigued, faint (syncope) or unconscious.
Treatment Outline
- The extent and nature of interventions in any given scenario will depend on the learning objectives of the simulation and the level of learners. Fluid replacement is necessary to restore adequate tissue perfusion and oxygen supply. For example, undergraduate nursing students may wish to focus on the identification of fluid loss and administration of a unit of blood. More advanced practitioners may want to focus on fluid volume responsiveness.
- For blood loss – rapid replacement of blood volume
- Packed red blood cell transfusion (see Blood Transfusion, Documents and Excel Labels for Simulation in Healthcare – Includes Template)
- Blood plasma transfusion.
- Platelet transfusion
- For fluid loss
-
- Intravenous crystalloids.
- Treatment of underlying cause to prevent more blood loss.
- Inotropes (medications that increase force of heart contraction) – only in certain situations
-
Desired Patient Outcomes
- Patient alert and oriented
- Skin warm and dry
- Peripheral pulses strong
- Urine output 30 ml/hr or 0.5 to 1 ml/kg/hr
- Hct – 32%
- Systolic blood pressure 90 to 120 mm Hg
- Mean arterial pressure 70 to 105 mm Hg
- Cardiac index 2.5 to 4 l/min/m2
- O2 sat ?95 %
Simulation Setup Tips for Hypovolemia
Sponsored Content:
- Suggested patient scenarios and setup.
- GI bleed, trauma, burns, marathon runner, gastroenteritis, morning sickness (Hyperemesis Gravidarum)
- Internal hemorrhage set up – blood in stool, black tarry stool, vomiting blood, chest pain, abdominal swelling, blood in the urine.
- C/o muscle cramps, abdominal or chest pain (due to mesenteric or coronary ischemia)
- Some manikins can show cyanosis.
- C/o thirst, dry mouth.
- External hemorrhage moulage.
- Electronic health record with appropriate diagnoses such as history of peptic ulcer disease, collapse after extreme physical activity, first trimester pregnancy.
- Add anticoagulants to patient medication list – contributing factor to hemorrhage.
- Use confederates to indicate patient signs and symptoms that cannot be changed in a manikin.
- g. pallor, sweating (confederate can spray manikin with water droplets), restlessness.
- Add in diagnostics
- Labs:
- Elevated BUN, creatinine
- High or low sodium
- Increase lactic acid (note large GI loses can lead to alkalosis)
- Low hematocrit/hemoglobin with blood loss.
- Fluid loss can lead to hemoconcentration. Match labs to cause of blood or fluid loss.
- Fluid loss can cause changes in electrolytes depending on the cause.
- Fluid volume responsiveness. Identifying how much fluid is needed may be difficult and complex. For manikins with hemodynamic monitoring features.
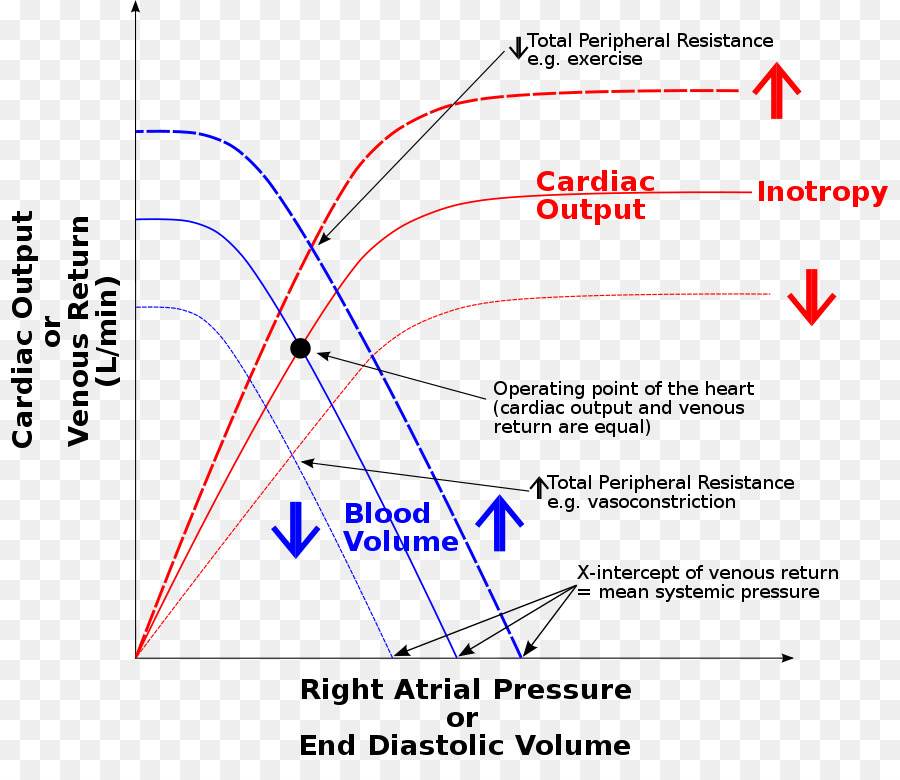
- Remember the Frank Starling curve. Within limits, stroke volume increases as cardiac preload increases.
- Stroke volume variation (SVV) with controlled mechanical ventilation may be measured.
- Physiology: Positive pressure breaths from a mechanical ventilator compress the superior and inferior vena cava in hypovolemic patients. This leads to a decrease in preload and ultimately a change in stroke volume and a swing in the arterial pressure tracing. An SVV of more than 13% indicates the patient is fluid responsive. Once the patient receives fluids and SVV is below 13%, further fluid will not be beneficial and inotropes may be considered (medications that increase blood pressure).
- There are limits to the use of SVV including lack of spontaneous breathing. Note this is only possible for manikins with the capability of showing stroke volume and cardiac output waveforms on a monitor.
- Passive Leg Raising (PLR)
- Pseudo fluid challenge of 150-300mL by placing patient/manikin in flat position and raising the legs 45 degrees. Blood moves from legs to the central core and, if the patient is responsive, preload and stroke volume will increase by 10%.
- Fluid is administered and the procedure repeated to see if the patient will respond to more fluid.
- In manikins with no hemodynamic tracings but with an arterial line, an increase in pulse pressure of 9%, will indicate fluid sensitivity.
- Blood pressure cuff alone. If PLR raises blood pressure by 17% (99% specific), patient shows fluid volume responsiveness.
- This procedure may not be appropriate in all patients (e.g. with trauma or location).
- An increase in SV of 10% after fluid administration may suffice.
- Foley catheter with scant dark colored urine.
- Let the manikin behavior show confusion, restless, c/o feeling faint.
- Adjust vital signs to severity of scenario blood loss and learner interventions.
- Limit learning objectives. Focus debriefing on these objectives.
- Labs:
All physiology is complex, however adding realistic physiological changes to a simulation can greatly add to the fidelity of a scenario, increase student learning and ultimately improve patient care.
Further reading comes from “Assessing volume status and fluid responsiveness in the emergency department” by David C. Mackenzie and Vicki E. Noble (Clin Exp Emerg Med. 2014 Dec; 1(2): 67–77. Published online 2014 Dec 31. doi: 10.15441/ceem.14.040):
Resuscitation with intravenous fluid can restore intravascular volume and improve stroke volume. However, in unstable patients, approximately 50% of fluid boluses fail to improve cardiac output as intended. Increasing evidence suggests that excess fluid may worsen patient outcomes. Clinical examination and vital signs are unreliable predictors of the response to a fluid challenge. We review the importance of fluid management in the critically ill, methods of evaluating volume status, and tools to predict fluid responsiveness.
Careful management of volume status and fluid administration is an important determinant of outcomes of the critically ill. Fluid responsiveness cannot be predicted on the basis of clinical examination. The best tools to predict volume responsiveness in ED patients use a passive leg raise maneuver to detect a change in a hemodynamic variable; the aortic blood flow, as determined by echocardiographic velocity-time integral, is most applicable to the emergency physicians. For practitioners with basic competence in point-of-care ultrasound, serial ultrasound of the heart, lungs, and IVC is the best tool to help guide fluid resuscitation and to direct transition away from fluid administration to other therapies.
Read the Entire Physiology: The “Force” Behind Healthcare Simulation HealthySimulation.com Article Series:
- Part 1: Blood Pressure
- Part 2: Heart & Respiratory Rate
- Part 3: Pulse Oximetry
- Part 4: Diabetes
- Part 4B: Hypoglycemia & Excel Template for Simulated EHR
- Part 4C: Insulin
- Part 5: Sepsis
- Part 6A: Hypovolemia (Intro)
- Part 6B: Hypovolemia (Treatment & Simulation Tips)
- Part 7A: IV Fluids & Bags
- Part 7B: IV Pumps & Site Access
- Part 7C: PCA for Pain
- Part 8: ABGs
- Part 9: Sepsis Labs
Sign Up for HealthySim’s Free Newsletter for More Great Sim Tips!
Have a story to share with the global healthcare simulation community? Submit your simulation news and resources here!
Dr. Kim Baily, MSN, PhD, RN, CNE has had a passion for healthcare simulation since she pulled her first sim man out of the closet and into the light in 2002. She has been a full-time educator and director of nursing and was responsible for building and implementing two nursing simulation programs at El Camino College and Pasadena City College in Southern California. Dr. Baily is a member of both INACSL and SSH. She serves as a consultant for emerging clinical simulation programs and has previously chaired Southern California Simulation Collaborative, which supports healthcare professionals working in healthcare simulation in both hospitals and academic institutions throughout Southern California. Dr. Baily has taught a variety of nursing and medical simulation-related courses in a variety of forums, such as on-site simulation in healthcare debriefing workshops and online courses. Since retiring from full time teaching, she has written over 100 healthcare simulation educational articles for HealthySimulation.com while traveling around the country via her RV out of California.
Sponsored Content: