Medical Tube Connectivity – Time to Update Your Healthcare Simulation Lab?
Many medical simulation laboratories begin life after receiving a one time funding to cover the cost of building the physical structure of the lab and the purchase of clinical simulation equipment such as Human Patient Simulators (HPS). Funding for ongoing maintenance, repair, and updating equipment may or may not be included. Simulation labs however, need to keep up with changing technology. This includes updating supplies as simple as tubing and tubing connections. Learners should use the same equipment as those found in the clinical setting. Using old equipment or outdated procedures leads to a confusing learning experience and potentially leads to medical errors. In that context, today Dr. Kim Baily PhD, MSN, RN, CNE helps us review our sim lab tubing following her similar articles:
In August of 2014, the Joint Commission issued Sentinel Event Alert No. 53 aimed at preventing and managing the risk of tubing misconnections. The alert included a literature search that featured 116 case studies involving misconnections directing enteral feeding solutions into IV lines. These adverse events resulted in 21 tragic preventable deaths. The alert describes common causes of connection-related injuries and suggested strategies for the launch of the new ISO connector standards. Connection injuries not only occur with enteral tubes but also with oxygen supply systems, intrathecal medication administration, automated blood pressure cuff tubes (air embolism) and arterial and venous monitoring and medication systems. Misconnections between systems can lead to fatal outcomes.
The Joint Commission recommended that facilities develop and implement organization-wide policies related to the replacement of tubing connections. The policies should include training and reporting near misses, unsafe conditions and adverse events. The Joint Commission devised a plan which included a number of phases for the safe development and implementation of new tubing connections over a number of years. And although the original announcement occurred in 2014, it is only recently that full implementation of the changes and training within US hospitals has begun. In July 2016, a California State Senate Bill No. 158 was introduced which prohibited “the use of intravenous, epidural, or enteral feeding connections that would fit into a connection port other than the type it was intended for.….”.
Sponsored Content:
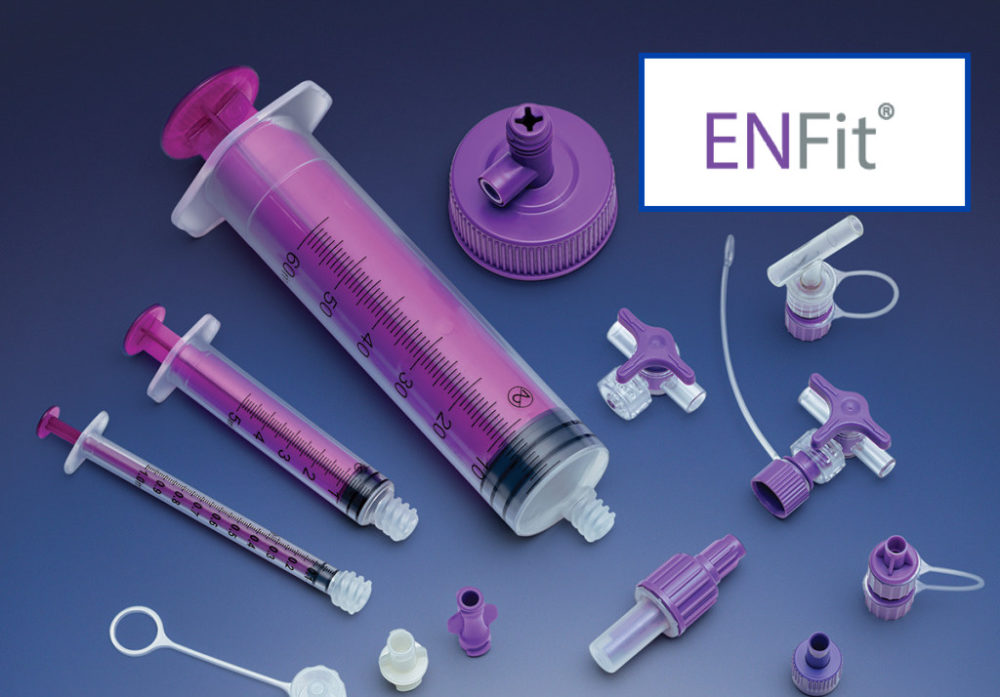
Although more than 80 California hospitals and other countries around the world have transitioned to use of ENFit connectors for enteral devices, fewer hospitals have adopted the standard across the rest of the US. Many are in the planning stages, realizing that full adoption is a tedious process and may take several months. On September 7, 2018 the FDA released a letter to manufacturers, healthcare professionals and hospital purchasing departments encouraging the use of 80369-3 or ENFit connectors. If local area hospitals or homecare agencies are using the new tubing connections, simulation laboratories should also be using the new equipment.
Since tubing misconnections occur throughout the world, the International Organization for Standardization (ISO) developed tubing connector standards/guidelines for medical equipment manufacturers around the globe. The first ISO standard (80369-1 General Standard) was followed by standards related to enteral connections. The old enteral connection was a “Christmas Tree” shape (male into female tube). The new connections named ENFit connector are designed to create physical barriers that make incorrect connections impossible.
The ENFit is made of a harder plastic and the connection itself has been reversed i.e. the administration set and syringes have a female end and the enteral tube itself has a male connection. An ENFit transitional connected was developed which can be used with older style enteral tubes. These are expected to be phased out once all patients have tubes with new connections. The next phase of full implementation includes the development of new neural tube connections. The Neuraxial (NRFit) system has a twist style connection but the NRFit tip is flush with the collar while the luer lock extends beyond the collar. This prevents the two systems from being connected. Some companies use colors to identify tubing for different systems but this has not been endorsed by The Joint Commission.
Sponsored Content:
The traditional luer lock connectors will be used exclusively for intravascular or hypodermic applications but intrathecal, enteral and limb cuff inflation devices should have different connectors which are unique to the individual system. Thus each system has a unique mechanical design which includes a connection which is incompatible with all other systems.
Safety Recommendations for Tubing:
As well as replacing old tubing with the new tubing and connectors, other safety recommendations include:
- Trace tubing or catheter from the patient to point of origin before connecting or reconnecting any device or infusion at any transition, such as to a new setting or service.
- Include tubing and connections in shift assessment and as part of the hand-off process.
- Standardize “line reconciliation” process using high reliability practices such as peer checking or peer coaching, and the STAR (Stop, Think, Act, Review) error prevention technique (the acronym is used to help remember to slow down and concentrate on an important action or task).
- Route tubes and catheters having different purposes in different, standardized directions (e.g., IV lines routed toward the head; enteric lines toward the feet). This is especially important in the care of neonates.
- When there are different access sites or several bags are hanging, tubing should be labeled to mitigate against the chance of misconnection, especially in circumstances where multiple IV lines are in use. Label tubing at both distal (near the patient connection) and proximal (near the source container).
- Ensure the implementation of safe practices for the administration of high-alert medications. For high-risk medications delivered via an epidural, intrathecal or arterial route, label the catheter and do not use tubing or catheters that have injection ports. Implement an independent double-check procedure to be used during the delivery of high-risk medications, such as intrathecal drugs, as well as during other procedures that have an increased frequency of adverse events.
- Use tubing and related equipment only as they are intended to be used.
- Never use standard luer syringes for oral medications or enteral feedings; use oral syringes for oral liquid medications or enteral feedings until enteral syringes with the new connector are available.
- Do not use IV tubing or IV pumps for enteral feedings.
- Use distinctly different pumps for IV applications (rather than using similar pumps for intrathecal and/or epidural applications) to reduce the possibility that an intrathecal medication will accidentally be delivered intravenously and vice versa.
- As soon as new connectors are available for the administration of intrathecal chemotherapy, use only syringes, needles and other devices with non-luer connectors that cannot connect to intravenous devices.
- Eliminate the use of temporary adapters as soon as possible.
- Do not force connections, and avoid workarounds. Forced connections or workarounds could indicate that the connection should not be made.
- Check vital signs immediately after making any connection.
- Take inventory and store carefully. Conduct an inventory of all supplies to identify products that need to be discontinued and products that need to be purchased when new ISO connector standards go into effect.
- Store medications for different delivery routes in different locations (e.g., keep intrathecal medications in a separate location from IV medications).
- Package together all parts needed for initiating enteral feeding, including tubing and catheters, to minimize the chance of using dissimilar tubes or catheters that could be connected improperly.
- Patient specific doses should be used where possible. They should be properly labeled and bar-coded for bedside scanning.
- Prescribers should include “via feeding tube” in orders.
Enteral Syringe Design: Making a Better Connection
Global Enteral Device Supplier Association (GEDSA) has worked with member companies to develop “low-dose” (small volume) ENFit tip syringes. These new syringes are incompatible with luer connectors and can be used to administer liquid medications either orally or via ENFit feeding tube connectors. The design is intended to address concerns about volume remaining in the syringe dead space when liquid medications are administered, potentially leading to dosage inaccuracy.
Compared with parenteral syringes, ENFit is a reverse gender system, with the feeding tube connector now male and the syringe connector now female. The low-dose syringe has a male design feature within the ENFit female connector (syringe tip) that fits inside the fluid lumen of the ENFit male connector on the feeding tube to prevent volume in the dead space. The new syringe fits ENFit straws and bottle adapters and can also be used to withdraw medication from a unit-dose cup. More information is available from the GEDSA website linked below.
The worldwide changes in tubing connections was the result of a collaboration between clinical, technical and regulatory agencies across the globe. The changes are designed to prevent misconnections and associated patient harm. Healthcare Simulation labs should ensure that they include the new equipment and safety standards where appropriate. During implementation of a new system, simulation can be used to as part of staff training. In an academic setting, deliberate errors in tubing connections or equipment can easily be incorporated into simulation scenarios to give learners the opportunity to routinely check tubings and connections to ensure connections are appropriate.
Visit the StayConnected Website to Learn More!
Have a story to share with the global healthcare simulation community? Submit your simulation news and resources here!
Dr. Kim Baily, MSN, PhD, RN, CNE has had a passion for healthcare simulation since she pulled her first sim man out of the closet and into the light in 2002. She has been a full-time educator and director of nursing and was responsible for building and implementing two nursing simulation programs at El Camino College and Pasadena City College in Southern California. Dr. Baily is a member of both INACSL and SSH. She serves as a consultant for emerging clinical simulation programs and has previously chaired Southern California Simulation Collaborative, which supports healthcare professionals working in healthcare simulation in both hospitals and academic institutions throughout Southern California. Dr. Baily has taught a variety of nursing and medical simulation-related courses in a variety of forums, such as on-site simulation in healthcare debriefing workshops and online courses. Since retiring from full time teaching, she has written over 100 healthcare simulation educational articles for HealthySimulation.com while traveling around the country via her RV out of California.
Sponsored Content: