Healthcare Simulation Conferences & Workshops
Healthcare Simulation conferences are an absolute must for professionals seeking to start or expand their institution’s simulation programs. As there are very few educational pathways for Medical Simulation professionals, participating in simulation in healthcare conferences like SimGHOSTS, IMSH or INACSL is a crucial step in developing or expanding your institution’s program and developing your professional career.
There are plenty of fantastic global conferences coming up in 2024 that provide a range of focused simulation learning, networking, and vendor engagement opportunities, so save the dates for these special events listed below. Previous annual events are summarized in the drop-down fields at the bottom of the page.
Don’t See Your Event Listed below? Email us to have your healthcare simulation conference listed.
Sponsored Content:
2024 Medical Simulation Conferences & Workshops
Featured Media Partnership Listings:
Human Patient Simulation Network (HPSN) Conferences
Various Dates & Countries Each Year
CAE’s largest Human Patient Simulation Network conferences are held throughout the year. They include premiere patient simulation events and networking conferences all over the world. Each meeting is uniquely designed to bring end-users, vendors, simulation program managers, medical professionals, trainees, and specialists together with thought leaders, influencers, and innovators in the healthcare simulation industry. Whatever a professional’s expertise, discipline, or clinical niche, there’s certain to be an event they won’t want to miss!
Sponsored Content:

Laerdal Medical’s Regional SUN Events
Various Dates & Countries Each Year
The Simulation User Network (SUN) Conference draws over 300 user network members to each meeting. It serves as a platform for collaboration, innovation and simulation advancements that lead to improvements in education and healthcare delivery. The SUN Conference is a place to meet others who have objectives in simulation just like yours and connect with industry-leading experts. You’ll experience best practices and hands-on demonstrations that help advance simulation training programs across all healthcare disciplines.

UPMC WISER Simulation Center Courses
(Administration, Sim Tech Ops, Debriefing, Center Design & More)
Various Dates Throughout the Year
The Winter Institute for Simulation, Education, and Research (WISER) is a world-class multidisciplinary training and research facility supporting education, training, and patient safety initiatives at UPMC. WISER is an institute of the University of Pittsburgh with a mission to conduct research and training programs utilizing simulation-based education to provide a safer environment for patients of the University of Pittsburgh Medical Center and its affiliates. WISER offers several courses and programs to help those in the simulation community improve their skills. Their iSIM course is offered in various worldwide locations and has had preceptors from all over the world spend time watching and learning at WISER.

NLN Education Summit 2024
September 18-20, 2024
San Antonio, Texas
The NLN Education Summit brings together nurse educators from around the world to explore trending and emerging topics impacting the nursing education profession and to discover innovative strategies and solutions that address day-to-day challenges. Having demonstrated exceptional flexibility, adaptability, and courage, nurse educators continue to be called to engage local, national, and global communities, inspire others to act, and build partnerships that co-create health equity and social justice. This Summit will examine nursing’s past, make connections to the present, and lead nursing’s future as the NLN reimagines nursing education.

SimGHOSTS Hands-on Simulation Technology Conferences
SimGHOSTS Events
August 6-9, 2024 Indianapolis, Indiana
Congreso Internacional Simulacion En Salud
May 30 – June 1, 2024
Ciudad de Buenos Aires, Argentina
Join hundreds of Simulation Champions from around the world at our annual international hands-on training events! The “Gathering of Healthcare Simulation Technology Specialists” which will be operating in its eighth year as a US-based non-profit 501(c)3 organization based out of Las Vegas, Nevada. SimGHOSTS events provide a meeting place for you to exchange ideas and network with technical peers as well as receive specialized training in the design, selection, and application of healthcare simulation technologies. You will also have opportunities to meet with simulation-based vendors to engage with the latest in healthcare education technology.
 Society in Europe for Simulation Applied to Medicine (SESAM) Annual Meetings
Society in Europe for Simulation Applied to Medicine (SESAM) Annual Meetings
SESAM Prague 2024
June 19 – 21, 2024
Prague, Czech Republic
Congreso Internacional Simulacion En Salud
May 30 – June 1, 2024
Ciudad de Buenos Aires, Argentina
SESAM’s mission is to encourage and support the use of simulation in healthcare for the purpose of training and research. SESAM’s core mission is to develop a sustainable inter-professional community of practice across Europe that strives to advance knowledge, improve quality and promote access to healthcare simulation. SESAM’s vision is improved healthcare through simulation. Enabling safe, patient-centred care delivered by a competent and confident healthcare workforce in a well-functioning healthcare system. In order to achieve their mission and vision, they have developed a strategy with 5 strategic goals, each with specific objectives and implementation plans. It is envisioned that the strategy will be reviewed annually at the SESAM Annual Retreat to ensure it remains fit for purpose and responds to new challenges. SESAM will host the organization’s 28th Annual Meeting of the Society from June 14 to 16, 2023. The meeting will take place face-to-face in Libson, Portugal.
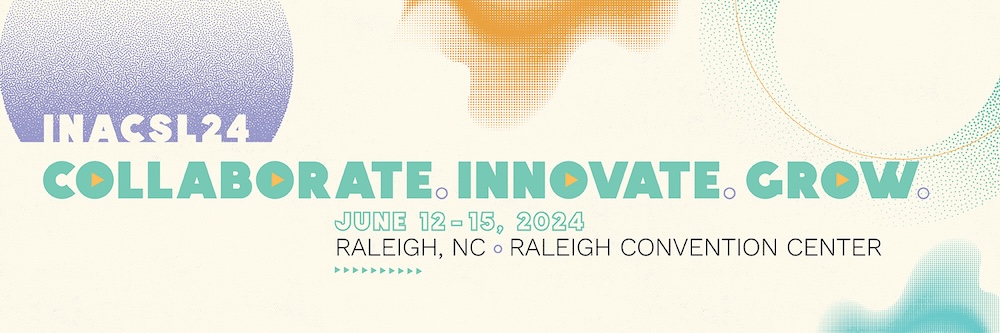
International Nursing Association for Clinical Simulation and Learning (INACSL) Conference 2024
June 12 – 15, 2024
Raleigh, NC
The INACSL Conference is a leading forum for simulation aficionados, researchers, and vendors providing the ideal environment to gain and disseminate current, state-of-the-art knowledge in the areas of skills/simulation operations and applications in an evidenced-based venue. Healthcare professionals will have the opportunity to network with colleagues and exhibitors, discuss best practices as they relate to competencies, safety, and quality performance indicators, and advance the science of simulation.

iSIM Courses
By UPMC WISER, UM GCRME, and UH SimTiki
These 3-day internationally renowned workshop programs, created in a collaborative effort between WISER at the University of Pittsburgh and the Gordon Center for Research in Medical Education at the University of Miami, is designed as an introduction to fundamental skills and abilities for delivering simulation-based healthcare education through a variety of techniques and technologies. The program emphasizes hands-on activities and active participation to maximize simulation-based instruction skill acquisition. Class group sizes are kept small to allow for maximum participation. The primary audience for this course is healthcare educators wishing to improve their skills as instructors in simulation education.
 International Society for Quality in Health Care
International Society for Quality in Health Care
September 24 – 27, 2024
Istanbul, Turkey
ISQua conferences provide a platform for like-minded peers to network, share their ideas, and foster innovation all in the hope to improve and support safe health care worldwide. ISQua is a member-based, not-for-profit community and organisation dedicated to promoting quality improvement in health care. They have been working to improve the quality and safety of health care worldwide for over 30 years. They aim to achieve their goal through education, knowledge sharing, external evaluation, supporting health systems worldwide and connecting like-minded people through their health care networks. Their extensive network of health care professionals spans over 70 countries and 6 continents. ISQua’s members are continually working towards quality improvement in health care around the world.
November 25 & 26, 2024
Fairmont Banff Springs
SIM Expo continues to be the largest interprofessional and multidisciplinary simulation conference in Canada! Each year, the SIM Expo brings together healthcare leaders in university, college, hospital, emergency services, and community care sectors. Their participants are deans, administrators, educators, clinicians, SIM centre managers and directors, and researchers from all health professions. Most participants are Canadian but it is an open conference and each year they are honored to host some international participants.
New Zealand Association for Simulation in Healthcare Conference
November 14 & 15, 2024
Christ Church, New Zealand
NZASH was incorporated as a Society in 2004 after an initial simulation interest group meeting in Hamilton, New Zealand. In brief, the Society aims to provide: a coordinating network for collaboration on healthcare simulation activities in NZ, including research representation of the views of members on healthcare simulation educational support and resource information for providers of healthcare simulation, including educational meetings a forum for national issues including standards, funding and other associated matters.
Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference
November 3-5, 2024
Edinburgh International Centre
ASPiH is a non-profit membership association comprising of members drawn from healthcare, education, and patient safety backgrounds including researchers, learning technologists, workforce development or education managers, administrators, and healthcare staff and students. All have a genuine interest in new and innovative methods of learning, as well as optimizing the use of existing simulation resources within their practice. Their membership bridges undergraduate and pre-registration education as well as postgraduate and post-registration training and ongoing CPD for all of the health and social care workforce. Dates for the organization’s 2022 annual event have not yet been announced, but HealthySimulation.com will post details as soon as they’re available.
 International Pediatric Society for Simulation (IPSS) Symposium
International Pediatric Society for Simulation (IPSS) Symposium
May 7-9, 2024
Denver, Colorado
Renowned global experts gather annually for the world’s largest meeting dedicated exclusively to pediatric and perinatal simulation. Over three days, attendees discuss the role simulation plays to provide safe and effective care to sick children and infants, and the continued evolution and expansion of pediatric simulation across the globe. Participants can learn about the latest advancements in pediatric and neonatal simulation from experts and see the highest quality simulators in the market. Dates for IPSSW2022 have not yet been announced, but HealthySimulation.com will post details as soon as they are available.
 Simulation Australasia
Simulation Australasia
2024 Events to be Announced
Simulation Australasia brings together industry professionals, government, and those interested in Simulation to allow for discussion and distribution of information through events and collaboration. The organization promotes the advancement of research, development, and use of simulation technologies and practices in society, industry, academia, and government. Simulation Australasia also advocates for standards and recognition of core competencies to enhance credibility and build trust. The organization promotes continuous research and development of modeling and simulation capabilities to foster innovative, and safer communities
8th Annual Virtual Reality and Healthcare Global Symposium
February 29 – March 3, 2024
Tampa Bay, Florida
This conference will be held at Penn Medicine (3400 Civic Center Blvd.) and will provide a forum to collaborate and partner with healthcare leaders to enable the creation of larger data sets. The goal is to foster partnership and collaboration in healthcare while providing a strong focus on providing unique content and perspectives from across the healthcare spectrum. There will also be a strong focus on providing new emerging voices from across the ecosystem: care, technology, research, and policy.
Operational Medicine Symposium & Technology Showcase
March 25 – 26, 2024
San Antonio, TX
The 5th Annual Operational Medicine Symposium (OpMed) & Technology Showcase is one of the largest and most influential military medical events in North America for senior medical officials, clinicians, government leaders, and solution providers. OpMed offers the unique opportunity to learn, network, and engage with military medical professionals in an information-driven, networking-focused environment.
California Simulation Alliance (CSA)
Various Workshops & Conferences Throughout the Year
In 2008, HealthImpact founded the California Simulation Alliance (CSA) to network and support interdisciplinary healthcare educators in California and beyond. Increasingly, healthcare professionals are utilizing multiple types of simulation in pre-licensure schools to provide a high-quality clinical experiences and in hospitals to improve patient care and teamwork. The CSA’s mission is to strengthen expertise in simulation and advocate for simulation policy through interprofessional education, collaboration, and evidence-based practice

11th Annual World Patient Safety, Science & Technology Summit
September 6-7, 2024
University of California, Irvine Campus
Every year, the Patient Safety Movement Foundation convenes the World Patient Safety, Science & Technology Summit, a global conference on patient safety featuring multidisciplinary sessions and keynotes by renowned experts in their fields. Every year, the Patient Safety Movement Foundation convenes the World Patient Safety, Science & Technology Summit, a global conference on patient safety featuring multidisciplinary sessions and keynotes by renowned experts in their fields.

SIMPACT 2024
May 20 -21, 2024
Aga Khan University, Karachi, Pakistan and Aga Khan University, Nairobi, Kenya
Welcome to SIMPACT: New Horizons in Simulation! SIMPACT 2024 is AKU’s first-ever international conference dedicated to harnessing the power of simulation-based education for a safer, more skilled healthcare future from May 20th to 21st, 2024. We believe in fostering a vibrant community of simulation professionals – students, educators, experts, and industry leaders – united by a shared goal: advancing patient care and promoting patient safety.
2024 General Conference Listings:
-
- IMSH 2024: January 20-24, 2024 – San Diego, CA
- Morocco Sim Congress Accueil | HTIC 2024 Fez: February 22-24, 2024
- ACS Surgical Simulation Summit – March 14 – 15, 2024 – Swissotel, Chicago
- IVRHA 2024: February 29 – Mar. 3, 2024 – Tampa Bay, Florida
- 8th Annual Lean Healthcare Research Symposium: March 21, 2024 – Carlsbad, California
- Human Patient Simulation Network (HPSN) Conferences from CAE Healthcare – Various Dates
- International Symposium on Human Factors and Ergonomics in Health Care: March 24 – 27, 2024 – Chicago, Illinois
- Operational Medicine Symposium & Technology Showcase – March 25-26, 2024 – San Antonio, Texas
- Virtual Medicine 2023 by Cedars Sinai Virtual Medicine: March 28-29, 2024 – Sofitel Hotel, Los Angeles, California
- NPOC (Nursing Practice Oversight Course): April 3, 2024 – Hilton Orlando – 6001 Destination Parkway Florida Ballroom Orlando, FL 32819
- XR EXPO 2024: April 3-4, 2024 – Willi-Bleicher Straße 19, Stuttgart, Baden-Württemberg, DE, 70174
- 2024 National Nurse Educator Summit – April 7-10, 2024 – Salt Lake City, Utah
- Can-Sim (Canadian Alliance of Nruse Educators Using Simulation) – April 9-10, April 13 and April 25 – Online via Zoom
- International Congress on Academic Medicine (ICAM) April 12–15, 2024 – Vancouver, Canada
- EMS Today The JEMS Conference and Exhibition – April 16-17, 2024 – JW Marriott Indianapolis
- DMMSO Medical Modeling, Simulation, & Pre-Hospital Innovation Expo: April 18, 2024 – 1395 Chaffee Road San Antonio, TX 78234
- Simulation Expo 2024: April 19, 2024 – LSUHSC Human Development Center – 411 South Prieur Street, 1st Floor New Orleans, LA 70112
- Virtual Reality Simulation for Medical Educators – April 19, 2024 – Worcester College, Oxford
- Trends in Nursing Education: AI, XR, CBE, Whaaaaat?! – April 25, 2024 – Virtual Event
- The International Nursing Conference – April 24-25, 2024 – Riyadh, Saudi Arabia
- 2024 Safar Symposium – April 25-26, 2024 – University of Pittsburgh School of Medicine
- AUK Simulation Conference – Iraq, TBD
- IPSSW 2024 – May 7-9, 2024 – Denver, Colorado
- Charles Morris Ginsburg Simulation-Based Quality Improvement and Research Forum – May 8, 2024
- Mental Health Simulation Conference 2024 – May 9, 2024 – 82-96 Grove Lane, London, SE5 8SN
- SOMA 2024 – May 13-17, 2024 – Raleigh, NC
- WCCHSE 2024 Conference – May 16 – 17, 2024 – BCIT Burnaby Campus
- SIM 4 SAFETY CONFERENCE – May 22 – 23, 2024 – Southampton Solent University
- IAS SYMPOSIUM 2024 – May 24, 2024 – University of Galway, University Road, Galway, Ireland H91 TK33
- Euroanaesthesia 2024 – May 25 – 27, 2024 – Munich, Germany
- Congreso Internacional Simulacion En Salud – May 30 – June 1, 2024 – Ciudad de Buenos Aires, Argentina
- ACH 2024 ENRICH Healthcare Communication Course – May 30 – June 2, 2024 – The Westin Peachtree Plaza Atlanta, GA
- Simulation Training for the Healthcare Professional – (Course) June 3 – 6, 2024
- Maryland Community and College Simulation Users Network Annual Conference – June 4 – 5, 2024 – Baltimore, Maryland
- AWE VR Events – June 18 – 20, 2024 in Long Beach and International Conference Dates
- Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN): June 7 – 11, 2024 – Phoenix, AZ
- Virtual Care Congress – June 11, 2024 – St. Georgen in the Black Forest
- Colloque simulation en santé: June 11-12, 2024 – Joliette, Canada
- SIM-MIDWEST Regional Conference – North Dakota, TBD
- INACSL 2024 : June 12 – 15, 2024 – Raleigh, NC
- SIMOPS 2024: July 17-19, 2024 – Aurora, Colorado
- SESAM Prague 2024: June 19 – 21, 2024 – Prague, Czech Republic
- 2024 ASPE Annual Conference: June 23 – 26, 2024 – Vancouver, British Columbia, CA
- Simulation In Motion Midwest: June 27 – 28, 2024 – Iowa City, IA
- VR/AR Association Virtual Healthcare Forum – TBD
- Nurse Educators Conference in the Rockies – July 24-28, 2024 – Beaver Run, Colorado
- VASSA Conference – July 29-31, 2024 – Great Wolf Lodge, Williamsburg, VA
- SimGHOSTS USA 2024 – August 6-9, 2024 – Indianapolis, Indiana
- SIMex Mexico – TBD
- BC Simulation Network Conference: TBD
- Simulation Australasia ASC: TBD
- Serious Play Conference 2024: August 12 – 14, 2024 Toronto, Canada
- 11th Annual World Patient Safety, Science & Technology Summit – September 6-7, 2024 – University of California, Irvine Campus
- 2023 SimTech Up: September 9th-11th, 2024 in Lubbock Texas
- ASPIRE: September 9 – 13, 2024 – Phoenix, Arizona
- ISAM 2024: September 11-13, 2024 – Sheffield, UK
- NLN Education Summit 2024 – September 18-20, 2024 – San Antonio, Texas
- Johnson County Community College Healthcare Simulation Conference: September 20, 2024 – Overland Park, KS
- ISQua 2024: September 24 – 27, 2024 – Istanbul, Turkey
- Healthcare Simulation In Person 2024 Conference: Elements, Essentials & Excellence in Healthcare Simulation: September 26, 2024- September 27, 2024 – Hilton Rochester Mayo Clinic Area
- Saudi Society for Simulation Scientific Assembly – TBD
- Immerse Global Summit Orlando 2024: TBA
- The International Symposium on Academic Makerspaces (ISAM) 2024: September 11th, 2024 – Sheffield, UK
- Utah Simulation Coalition Conference 2024: October 5, 2024
- WISER/UPMC Symposium on Rapid Response Systems – TBD
- Academic Nursing Leadership Conference: October 12-14, 2024 – Washington, D.C.
- Global Network for Simulation in Healthcare: October 24 – 27, 2024 – Toronto, Canada
- Association for Simulated Practice in Healthcare UK: November 3-5 – Edinburgh International Conference Centre
- SimONE SIM Expo – November 25 – 26, 2024 – Fairmont Banff Springs Alberta, Canada
- 1st Arab Resuscitation Council Conference – TBD
- Simulation Canada Sim Expo 2024: TBA
- Congrès 2023 de la SoFraSimS: TBD
- Australasian Simulation Congress : August 11-14, 2025 Adelaide
2024 General Workshop Listings:
-
- Introducing the Future of Anatomy Webinar Series – February 14, March 19, April 24, 2024
- Sim Summit Atlanta – March 22, 2024 – 250 E. Ponce de Leon Avenue, Decatur, GA 30030
- Supporting Newcomer Health: Enhancing Your Skills with Interpreters Workshop – April 2 – 3 and April 15, 2024
- IPSS Academy: Simulation Design and Practice – May 10, 2024
- Neonatal Simulation Instructor Course – June 25 – 26, 2024 – Chilworth Southampton United Kingdom
- STRATUS Instructor Training Seminars – TBD
- One-Sim Program (Obstetrics and Neonatal Emergency Simulation) – August 16, 2024
- CAMES Surgical simulation Masterclass: August 19 – 23, 2024 – Copenhagen, Denmark
- Multidisciplinary Healthcare Simulation – PGCert – TBD
- Military Moulage Workshops: Various Dates & Locations
- Moulage Concepts Workshops Around the United States: Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) – Various Dates and Locations
- CAN-Sim Virtual Simulation Design Workshop – Various Dates – Ottawa Ontario, Canada
- Boston Children’s Hospital: Simulation Courses – Various Dates – Boston, MA
- Jump Simulation Workshops – Various Dates – Peoria, IL
- California Simulation Alliance Education Courses – Various Dates around California
- Saviour Medical – Various Moulage and Medical Simulation Workshops – Various Dates
Don’t see your listing or want to be featured?
Email us the clinical simulation event title, dates, location, link, and that you would like to be featured!
2023 General Conference Listings:
-
- IMSH 2023: January 21-25, 2023 - Orlando, Florida
- SIM Summit Seattle - February 17th, 2023 - Seattle University Student Center 130
- ACS Surgical Simulation Summit - March 2-4, 2023 - Chicago, IL
- IVRHA 2023: March 3-5, 2023 - Philadelphia, PA
- Healthcare Simulation Conference 2023: Elements, Essentials & Excellence - March 9, 2023 - Rochester, Minnesota
- Human Patient Simulation Network (HPSN) India - March 17-19, 2023 - Medical Simulation Center VASA (Vydehi Advanced Simulation Academy), Bangalore, Indien
- Staffordshire University Education and Research Conference 2023 - March 28 to March 29, 2023 - Birmingham, United Kingdom
- Operational Medicine Symposium & Technology Showcase - March 28-29, 2023 - San Antonio, TX
- Virtual Medicine 2023 by Cedars Sinai Virtual Medicine: March 30-31, 2023 - Sofitel Hotel, Los Angeles, California
- Nursing Education Research Conference (NERC) - March 30-April 1 - Washington, D.C.
- International Congress on Academic Medicine (ICAM) April 13th – 18th, 2023 - Québec City, Canada
- 2023 National Nurse Educator Summit - April 24-27 - La Cantera Resort and Spa
- EMS Today The JEMS Conference and Exhibition - April 25-26, 2023 - Indianapolis, IN
- AUK Simulation Conference - April 28-29, 2023 - Duhok, Kurdistan Region of Iraq
- Virtual Care Congress - May 4, 2023 - Online Event
- SOMA 2023 - May 15-19, 2023 - Raleigh, NC
- IPSSW - May 17-19, 2023 - Lisbon, Portugal
- UT Southwestern Simulation Center Quality Improvement and Research Forum - May 17, 2023
- 2023 Safar Symposium - May 18-19, 2023, Pittsburgh, PA
- International Symposium on Human Factors and Ergonomics in Health Care: March 24 - 27, 2024 - Chicago, Illinois
- Euroanaesthesia 2023 - June 3-5, 2023 - Glasgow, Scotland
- 2023 ASPE Annual Conference: June 4-7, 2023 - Portland, Oregon
- Maryland Community and College Simulation Users Network Annual Professional Development Conference - June 6-7, 2023 - Coppin State University (Baltimore, Maryland)
- SIM-MIDWEST 4th Regional Conference - June 9-10, 2023 - Sioux Falls, South Dakota
- SESAM Lisbon 2023: June 14-16 2023 - Lisbon, Portugal
- INACSL 2023 - June 14-17, 2023 - Providence, Rhode Island
- Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN): June 17-21, 2023 - New Orleans, LA
- VR/AR Association Virtual Healthcare Forum 2023 - June 29 - Virtual Event
- The First International Conference on Simulation and Virtual Reality in Medicine: July 6-8, 2023 - Targu Mures, Romania
- Nurse Educators Conference in the Rockies - July 10-14, 2023 - Breckenridge, Colorado
- SIMOPS 2023 & SIMOPS DELIVERS: July 19-21, 2023 - Providence, RI
- SimGHOSTS USA 2023 - August 1-4, 2023 - Omaha, Nebraska
- SIMex 2023 - August 9-11, Facultad De Medicina at UNAM, Mexico City, Mexico
- BC Simulation Network Conference: August 17, 2023, Kamloops, Canada
- Simulation Australasia ASC 2023: August 21-24, 2023 - Adelaide, Australia
- 2023 SimTech Up: August 22-24, 2023 - Lubbock, TX
- ISQua 2023: August 27-30 2023 - Seoul, South Korea
- 2023 CAE HPSN Pacific NW Conference - Sept 8, 2023 - Olympic College - Bremerton, WA
- SWFL Simulation Conference - September 15, 2023 - Florida Southwestern State College 8099 College Parkway - Building AA Ft. Myers, FL 33919
- Saudi Society for Simulation Scientific Assembly 2023 - Sept 18 - 20, 2023, Dammam, Saudi Arabia
- SIM Summit Washington D.C. - September 22, 2023, George Washington University, Innovation Hall, 45085 University Drive, Ashburn, Virginia 20147
- Johnson County Community College Healthcare Simulation Conference: September 22, 2023 - 12345 College Blvd. Overland Park, KS 66210
- NLN Education Summit - September 28-30, 2023 - Washington, D.C.
- ISAM 2023: October 18-20, 2023 - Pittsburgh, PA
- SimHuddle - Collaborate, Connect & Chat: October 18 - October 20, 2023 / Honolulu, Hawai'i
- Immerse Global Summit Orlando 2023: October 17-19, 2023
- The International Symposium on Academic Makerspaces (ISAM) 2023: October 18-20, 2023 - Pittsburgh, PA
- USC Fall Conference 2023: October 21, 2023 - 12257 Business Park Drive Draper, Utah
- WISER/UPMC Symposium on Rapid Response Systems - October 26, 2023 - Cumberland Woods (UPMC Passavant Campus)
- Echo Healthcare & Concordia University Simulation Workshop - October 27, 2023 - Concordia University, 11703 NE Glenn Widing Drive Portland, OR
- Academic Nursing Leadership Conference: October 28-30, 2023 - Washington, D.C.
- Association for Simulated Practice in Healthcare UK: November 6th-8th, Brighton, England
- Global Network for Simulation in Healthcare: November 9th-12th, Dubai, UAE
- Asia Pacifics Premier XR Medical Conference: November 10-11,2023 - Sunshine Coast, Australia
- 1st Arab Resuscitation Council Conference - November 24-26, Dubai, United Arab Emirates
- Sim Expo 2023: December 11 & 12, 2023, in Ottawa, Ontario
- Congrès 2023 de la SoFraSimS: TBD
- Serious Play Conference 2023: Oct 11-13, 2023
- ISAM 2023: October 18-20, 2023 - Pittsburgh, PA
- SimHuddle - Collaborate, Connect & Chat: October 18 - October 20, 2023 / Honolulu, Hawai'i
- Immerse Global Summit Orlando 2023: October 17-19, 2023
- The International Symposium on Academic Makerspaces (ISAM) 2023: October 18-20, 2023 - Pittsburgh, PA
- USC Fall Conference 2023: October 21, 2023 - 12257 Business Park Drive Draper, Utah
- WISER/UPMC Symposium on Rapid Response Systems - October 26, 2023 - Cumberland Woods (UPMC Passavant Campus)
- Echo Healthcare & Concordia University Simulation Workshop - October 27, 2023 - Concordia University, 11703 NE Glenn Widing Drive Portland, OR
- Academic Nursing Leadership Conference: October 28-30, 2023 - Washington, D.C.
- Association for Simulated Practice in Healthcare UK: November 6th-8th, Brighton, England
- VR Healthcare Demo Tour: November 7-9,2023 - Various Locations
- Global Network for Simulation in Healthcare: November 9th-12th, Dubai, UAE
- Asia Pacifics Premier XR Medical Conference: November 10-11, 2023 - Sunshine Coast, Australia
- 1st Arab Resuscitation Council Conference - November 24-26, Dubai, United Arab Emirates
- Sim Expo 2023: December 11 & 12, 2023, in Ottawa, Ontario
2023 General Workshop Listings:
-
- Neonatal Simulation Instructor Course - February 8-9, 2023 - Chilworth Manor Hotel Chilworth Southampton United Kingdom
- STRATUS Instructor Training Seminars - 3/3-4/1/2023
- HPSN India 2023 "Obstetrical & Neonatal Journey - An Inclusive Simulated Clinical Experience" - March 17, 2023 - Vydehi Advanced Simulation Academy (VASA) - Karnataka, India
- Colloque simulation en santé: June 1-3, 2023 - Joliette, Canada
- CAMES Surgical simulation Masterclass: August 21-25, 2023 - Copenhagen, Denmark
- Multidisciplinary Healthcare Simulation – PGCert – Start Date in October 2023
- Military Moulage Workshops: Various Dates & Locations
- Moulage Concepts Workshops Around the United States: Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) – Various Dates and Locations
- George Washington University Simulation Workshops – Various Dates in Washington D.C.
- CAN-Sim Virtual Simulation Design Workshop - Various Dates - Ottawa Ontario, Canada
- Boston Children’s Hospital: Simulation Courses - Various Dates - Boston, MA
- California Simulation Alliance Education Courses – Various Dates around California
2022 General Conference Listings:
- Virtual International Meeting for Simulation in Healthcare 2022 (IMSH) by the Society for Simulation in Healthcare (SSH) - January 15 - 19, 2022 - Los Angeles, CA
- North America HoloLens Industry Summit - January 26 - 8 AM.- 1:15 PM / 11 AM - 4:15 PM EST, virtual
- International Conference on Simulation in Healthcare - TBD, India
- 6th Annual Virtual Reality and Healthcare Conference - March 3-4, 2022 - Nashville, Tennessee
- International Symposium on Human Factors and Ergonomics in Health Care - March 20-23, 2022 - New Orleans, LA
- Virtual Medicine 2022 by Cedars-Sinai Virtual Medicine - March 24, 2022, virtual
- Simulation Workshop: Initial Assessment and Resuscitation of the Seriously Ill Child (Focused PedSim) - June 6-7, 2022 - Riyadh Clinical Simulation Center, KASU-HS, Riyadh
- CALSIM 2022: Cedars-Sinai Annual Simulation Conference - April 1, 2022, 8AM to 2 PM PST - Cedars-Sinai Women’s Guild Simulation Center, Los Angeles, CA
- Getting WISER on Diversity, Equity, and Inclusion in Healthcare Education (The Roles of Simulation) - April 8, 2022 (virtual conference 8 AM to 12 PM EST / onsite workshop 1 PM to 5 PM EST)
- HealthySimulation.com Champions of Simulation Virtual Conference - April 13 , 2022 8AM-4PM PDT, UTC-7
- EMS Today The JEMS Conference and Exhibition – April 25-30, 2022 - Indianapolis, IN
- Healthcare Simulation In-Person Conference 2022: Elements, Essentials & Excellence in Healthcare Simulation - April 29-30, 2022 - Rochester, MN
- World Patient Safety, Science & Technology Summit - April 29-30, 2022, virtual
- UT Southwestern Simulation Center Quality Improvement and Research Forum - May 2022
- SOMA 2022 Scientific Assembly – May 2-6, 2022, Raleigh, NC
- AAPA Conference 2022 - May 21-25, 2022, JW Marriott Indianapolis, 10 S West St., Indianapolis, IN 46204
- 19th Safar Symposium, University of Pittsburgh School of Medicine – May 26-27, 2022
- Euroanaethesia 2022 – June 4-6, 2022 - Milan, Italy
- Simulation Conference - Linking Safety, Education and Practice - June 8, 2022, Centre for Health Innovation, Staffordshire University, Blackheath Lane, Stafford, ST18 0YG, United Kingdom
- 14th International Pediatric Simulation Symposia and Workshops "Exploring cultural dimensions of simulation: Diversity, Debrief, Disseminate" | June 10 - 13, 2022 (St. Petersburg, Florida)
- Serious Play Conference - June 13-17, 2022 - virtual/live (Orlando, Florida)
- SESAM Seville: Building Simulation for Health Challenges - June 15-17, 2022 - Seville, Spain
- INACSL22 - June 15-18, 2022 - Milwaukee, Wisconsin
- Congrès 2022 de la SoFraSimS – June 22-24, 2022
- AANP Conference 2022 - June 22-25, 2022, Orange County Convention Center, 9400 Universal Blvd, Orlando, FL 32819
- Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) – June 25-29, 2022 in Aurora, Colorado
- 2022 ASPE Annual Conference - June 26-29, 2022 - New Orleans, Louisiana, USA
- ACEN Nursing Education Accreditation Conference - July 13-15, 2022 - Atlanta, GA & Virtual
- SIMOPS 2022 & SIMOPS DELIVERS - July 20-22, 2022, Greater Cincinnati Area (St. Elizabeth Training and Education Center), virtual
- SimGHOSTS USA 2022 | Proudly hosted by Baylor University Louise Herrington School of Nursing - August 2-5, 2022 - Dallas, Texas
- SPSIM 2022 - August 31, 2022 - September 2, 2022 - Lausanne University Hospital (CHUV) in Lausanne, Switzerland
- SUN: Simulation User Network - September 12-14, 2022 - Houston, Texas
- 2022 SISO SIMposium - Sept 14-15, 2022 - virtual
- Johnson County Community College Healthcare Simulation Conference - September 16, 2022 - 12345 College Blvd., Overland Park, KS 66210
- NLN Education Summit - September 28-30, 2022 - Las Vegas, Nevada
- 2022 SIM EXPO - October 17-18, 2022 - Toronto, Ontario
- S3 Singapore 2022 - October 19-21, 2022
- 3rd Annual Healthcare Distance Simulation Summit - October 21st, 2022 from 9 am-1 pm EST -
- Academic Nursing Leadership Conference - October 25-November 1, Washington, DC, virtual
- ICGESS 2022: 16. International Conference on Game Engineering, Simulation and Simulator - October 28-29, 2022 Paris, France
- EMSUK Learning 2022 - October 29, 2022, Cardiff City Football Stadium, (Leckwith Rd, Cardiff CF11 8AZ, United Kingdom)
- ASPiH Conference 2022 - November 6-8, 2022, Birmingham, U.K.
- 5th Saudi Health Simulation Conference - November 06 - 10, 2022 - Riyadh, Saudi Arabia
- The International Symposium on Academic Makerspaces (ISAM) 2022 - November 6-9, 2022, virtual
- Columbia University School of Nursing's 4th annual Innovations in Simulation Conference - Friday, November 11, 8:30 a.m. to 5 p.m., The Forumat Columbia University, 601 W. 125th Street, New York, NY
- NZASH Conference 2022 - November 11-12, 2022, Te Auaha NZ Institute of Creativity, 65 Dixon Street, Wellington
- California Simulation Alliance Annual Conference - November 12, 2022, Cedar-Sinai in Los Angeles, CA
- SIMex- 2022- November 15-18, 2022, Mexico City, Mexico
- OADN 2022 Convention - November 18-20, New Orleans, LA
2022 General Workshop Listings:
- SESAM Winter School - January 7, 2022
- CHSE Blueprint Review Course (Led by Healthcare Simulation Experts at UCF) - Saturday, March 19, 2022, 8:30 a.m. – 5:00 p.m. EST - Online, Live Interactive Course (Taught via Zoom)
- Limmer Education PCRF Education Research Journal Club Webinar - "Hybrid Simulation: A Pathway to Airway Mastery?" March 25, 12-1 PM ET, virtual
- Colloque simulation en santé - June 2-3, 2022 in Joliette, Canada
- CAE SimEquip ANAESTHESIA - June 15, 2022, 7 PM IST
- Journal Club Debriefing - June 16, 2022 at 4 PM ESP, virtual
- CAMES Surgical simulation Masterclass - August 22-25, 2022, Copenhagen, Denmark
- Multidisciplinary Healthcare Simulation - PGCert - Start Date in October 2022
- Mental Health Simulation-Based Education Event - September 7 and October 5, 2022 (delivered by the S4MH team at CCCU as part of the HEE KSS Regional Simulation and Human Factors Project)
- Simulation Workshop: Initial Assessment and Resuscitation of the Seriously Ill Child - September 27-28, 2022 from 8 AM to 5 PM - Riyadh Clinical Simulation Center, KASU-HS, Riyadh
- Centre for Healthcare Simulation (CHS) at the NUS Yong Loo Lin School of Medicine | Debriefing in Simulation-Based Healthcare Education
- WISER Simulation Center Courses (Administration, Sim Tech Ops, Debriefing, Center Design & More) - Various Dates Throughout the Year
- iSIM Debriefing Course from WISER, SimTiki, and GCRME - Various Dates and Locations
- Military Moulage Workshops - Various Dates and Locations
- Moulage Concepts Workshops Around the United States - Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) - Various Dates and Locations
- Simulation Professionals of Texas (SPOT) Connections & Collaborations - Various Dates
- The Debriefing Academy Master Debriefer Course - Various Dates & Locations
- George Washington University Simulation Workshops – Various Dates in Washington D.C.
- Jump Simulation Courses: Instructor, Debriefer, and More - Various Dates
- Boston Children's Hospital: Simulation Courses - Various Dates in Boston, MA
- California Simulation Alliance Education Courses - Various Dates around California
- University of Illinois Professional Development Programs for Standardized Patient Educators - 2022 DATES TBA
- Level 3 Healthcare Sim Tech Bootcamp - 2022 DATES TBD
2021 Medical Simulation Conferences
*The following conferences may still be affected by COVID-19!*
- 2021 National League for Nursing Virtual Scholarly Writing Retreat: An NLN Mentoring Program - January 9-10, 2021, virtual
- Virtual International Meeting for Simulation in Healthcare 2021 (IMSH) by the Society for Simulation in Healthcare (SSH) (In person event canceled) - January 19th - March 31, 2021, Online
- Royal College of Physicians and Surgeons of Canada 2021 Simulation Summit - February 17-18, 2021, virtual
- International Virtual Reality Healthcare Association 5th Annual Virtual Reality and Healthcare Global Symposium - March 2-5, 2021, virtual
- Clinical Human Factors Group "The impact of staff well-being on creating safety" March 18, 2021, virtual
- CALSIM 2021 Inaugural Simulation Conference - April 9-10, 2021, virtual
- HFES International Symposium on Human Factors and Ergonomics in Health Care - April 12-16, 2021, virtual
- ASME Visualize MED Conference - April 14-15, 2021, virtual
- Society in Europe for Simulation Applied to Medicine (SESAM) Virtual Annual Conference - April 14-16, 2021, virtual
- 2021 Australasian Simulation Congress - May 4-7, 2021 in Brisbane, Australia
- IHI Patient Safety Congress - May 11-13, 2021 in San Diego, California
- 18th Safar Symposium, University of Pittsburgh School of Medicine - May 19-20, 2021, virtual
- Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) - June 12-16, 2021 in Orlando, Florida
- International Nursing Association for Clinical Simulation and Learning (INACSL) Hybrid 2021 Event - June 16-19, 2021 in Denver and Virtually Online
- 2021 Serious Play Conference - June 22-25, 2021, Virtual
- Congrès 2021 de la SoFraSimS - June 23-24, 2021, Virtual
- Association for Standardized Patient Educators Annual Conference (ASPE) - June 27-30, 2021, La Jolla, California
- SOMA 2021 Scientific Assembly - June 28-July 2, 2021, Charlotte, NC
- International Society for Quality in Health Care (ISQua) - July 8-11 in Florence Italy
- UK Patient Safety Congress – July 12-13, 2021 in Manchester Central, United Kingdom
- Society for Simulation in Healthcare SimOps - July 14-16, 2021, Tampa, Florida or virtual
- SimGHOSTS USA - August 3-6, 2021, Fort Wayne, Indiana
- EMS Today The JEMS Conference and Exhibition - August 24-27, 2021, San Antonio, Texas
- ISAGA2021: Gaming, Simulation and Innovations: Challenges and Opportunities - September 6 - 10, 2021, Indore, India and virtual
- Simulation Week 2021 Panel: The Future of Healthcare Simulation - September 14, 2021 at 12 PM EDT, Virtual (Facebook Live)
- Healthcare Simulation Week 2021 CSDS SIMinar Series: To celebrate the this year CSDS has brought back the popular webinar series #SIMinars! Sessions will run online for 30 to 60 minutes starting at 12 p.m. (AEST) from Monday September 13 until Friday September 17. These are free events and registration is open to all.
- Kansas City Healthcare Simulation Conference: September 17, 9 a.m. to 3 p.m., Johnson County Community College
(12345 College Blvd. Overland Park, KS 66210 Regnier Center 101) - Building a Sustainable Future: The 2nd Annual Telesimulation in Healthcare Conference, hosted by Weill Cornell Medicine NewYork-Presbyterian Simulation Center - September 23, 2021, Virtual
- NNBA Annual Educational Conference on Nurse Entrepreneurship and Career Alternatives - September 24-26, 2021, virtual
- SimforTalk 2021: La conférence annuelle de la simulation numérique en Santé - October 12, 2021 9:30 a.m. to 12 p.m.
- European Simulation Research Network (SiReN) - October 14, 2021, virtual
- Alabama Modeling & Simulation Council Virtual Healthcare Simulation Technologies Conference - October 21, 2021
- Australasian Simulation Congress - November 9-11, 2021 - Virtual
- 3rd Annual Virtual Reality and Healthcare Europe Conference - December 2-3, 2021 - Dublin, Ireland
- SIMULCON 2021 - December 3-5, 2021 from 0900 Hrs to 1600 hours (Indian Standard Time), virtual
- Saudi Society for Simulation Scientific Assembly 2021 - December 6-9, 2021
- European Society of Anaesthesiology Patient safety Policy Summit - December 17-19, 2021 in Munich
- SimGHOSTS-X One Day Event - Date TBD in 2021, UTHSC Memphis, Tennessee
- FDA Public Workshop on Simulation Practices in Health - Date TBD in 2021, Silver Spring, Maryland
General Workshop Listings:
- WPSSTS Panel: Healthcare Safety During the Pandemic | April 29-30 (8 a.m. to 3 p.m.), 2022, virtual
- Trauma Resuscitation in Kids (TRIK): Trauma Resuscitation in Kids (TRIK) is a two-day simulation-based course designed for health care providers who manage pediatric trauma patients. The course includes a mixture of simulation scenarios, group lectures, and technical skills sessions for practicing procedures. The format provides healthcare providers hands-on experience in a safe and experiential learning environment. (Thursday, September 8, 2022, 7:00 AM - Friday, September 9, 2022, 5:00 PM, Nationwide Children's Hospital Simulation Center, Columbus, OH)
- Centre for Healthcare Simulation (CHS) at the NUS Yong Loo Lin School of Medicine | Debriefing in Simulation-Based Healthcare Education
- WISER Simulation Center Courses (Administration, Sim Tech Ops, Debriefing, Center Design & More) - Various Dates Throughout the Year
- iSIM Debriefing Course from WISER, SimTiki, and GCRME - Various Dates and Locations
- Military Moulage Workshops - Various Dates and Locations
- Moulage Concepts Workshops Around the United States - Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) - Various Dates and Locations
- Simulation Professionals of Texas (SPOT) Connections & Collaborations - Various Dates
- The Debriefing Academy Master Debriefer Course - Various Dates & Locations
- George Washington University Simulation Workshops – Various Dates in Washington D.C.
- Jump Simulation Courses: Instructor, Debriefer and More - Various Dates
- Boston Children's Hospital: Simulation Courses - Various Dates in Boston, MA
- California Simulation Alliance Education Courses - Various Dates around California
- University of Illinois Professional Development Programs for Standardized Patient Educators - 2021 DATES TBA
- RUSH Simulation and Procedural Bootcamp for Emergency Medical Clinicians – DATES TBA
- Level 3 Healthcare Sim Tech Bootcamp - DATES TBA
2020 Medical Simulation Conferences & Workshops
Featured Media Partnership Listings:
 UPMC WISER Simulation Center Courses
UPMC WISER Simulation Center Courses
(Administration, Sim Tech Ops, Debriefing, Center Design & More) - Various Dates Throughout the Year
The Winter Institute for Simulation, Education, and Research (WISER) is a world class multidisciplinary training and research facility supporting education, training and patient safety initiatives at UPMC.. WISER is an institute of the University of Pittsburgh with a mission to conduct research and training programs utilizing simulation based education to provide a safer environment for patients of the University of Pittsburgh Medical Center and its affiliates. WISER offers several courses and programs to help those in the simulation community improve their skills. Their iSIM course is offered in various worldwide locations and have had preceptors from all over the world spend time watching and learning at WISER.

SimGHOSTS Hands-on Simulation Technology Conferences
Join hundreds of Simulation Champions from around the world at our annual international hands-on training events! The “Gathering of Healthcare Simulation Technology Specialists” which will be operating in its eighth year as a US-based non-profit 501(c)3 organization based out of Las Vegas, Nevada.
SimGHOSTS events provide a meeting place for you to exchange ideas and network with technical peers as well as receive specialized training in the design, selection and application of healthcare simulation technologies. You will also have opportunities to meet with simulation-based vendors to engage with the latest in healthcare education technology.
SimGHOSTS 2020 Annual Global Symposium
Converted to Virtual Event! More Info TBA:
August 4-7th
Baylor University
Dallas, TX
SimGHOSTS Saudi Arabia 2020
Princess Nourah University
February 26-28
One Day SimGHOSTS-X Event
POSTPONED April 3rd, UTHSC Memphis, Tennessee
 Society in Europe for Simulation Applied to Medicine (SESAM) Annual Conference
Society in Europe for Simulation Applied to Medicine (SESAM) Annual Conference
June 17-19 POSTPONED DUE TO CORONAVIRUS
Milan, Italy
SESAM's mission is to encourage and support the use of simulation in healthcare for the purpose of training and research. Join them in Milan, Italy from June 17-19 2020 for SESAM 2020. Discover the latest in healthcare simulation, network with colleagues and make new connections. Visit their industry partners at the exhibition area to find out about the latest advances in Simulation technology, be inspired by world-class speakers and stay ahead with cutting edge simulation research. They look forward to welcoming you to Milan for three days of invaluable learning and discovery!
 International Nursing Association for Clinical Simulation and Learning (INACSL) Conference 2020
International Nursing Association for Clinical Simulation and Learning (INACSL) Conference 2020
Converted to Virtual Event! More Info TBA: June 24-27, 2020
Raleigh, NC
Conference Location: Raleigh Convention Center
The INACSL Conference is a leading forum for simulation aficionados, researchers, and vendors providing the ideal environment to gain and disseminate current, state-of-the-art knowledge in the areas of skills/simulation operations and applications in an evidenced-based venue. Healthcare professionals will have the opportunity to network with colleagues and exhibitors, discuss best practices as relates to competencies, safety, and quality performance indicators, and advance the science of simulation.
![]() UIC Professional Development and Certificate Programs for Standardized Patient Educators
UIC Professional Development and Certificate Programs for Standardized Patient Educators
One-Week Intensive Program: Standardized Patient Educator Certificate Program – October 26-30, 2020
Two-Day Workshops : Special Topics in Instruction & Assessment with SPs – May 4-5, 2020
Training SPs to Debrief and Give Feedback – September 2-3, 2020
Learn to create compelling standardized-patient based educational programs to enhance health professions education. Designed for simulation educators in the health professions: Directors and staff of simulation and standardized patient (SP) centers Faculty who wish to leverage SPs to enhance their educational programs Intensive sessions focusing on key topics in SP-based education Small class sizes, highly interactive, abundant hands-on practice Sessions are led by Rachel Yudkowsky, MD, MHPE, Bob Kiser, CEC, CHSE, and faculty and staff of the Simulation and Integrative Learning Institute (SAIL) of the University of Illinois at Chicago College of Medicine. Founded in 1987 as one of the first standardized patient centers and now a comprehensive multi-modal simulation center, SAIL, formerly the Dr. Allan L. and Mary L. Graham Clinical Performance Center (GCPC), is internationally recognized for innovation in standardized patient education.
 CALSIM 2020 Inaugural Simulation Conference
CALSIM 2020 Inaugural Simulation Conference
POSTPONED until 2021 June 27-28 at Cedars Sinai in Los Angeles, CA
This engaging everything-you-need-to-know-about-simulation-based-training will provide participants with evidence-based presentations that explore how simulation can be an innovative tool for learning and training as well as for assessment of performance. Attendees will explore how simulation training can enhance technical and functional expertise, contribute to problem-solving and decision-making skills, and improve interpersonal and communication skills and team based competencies. Breakout sessions offer learners in-depth exploration of simulation design testing, human factors, and interprofessional education, as well as insights into the role simulation can have in infectious disease management and extracorporeal membrane oxygenation (ECMO). Join your colleagues for this 1-1/2 day adventure into the world of simulation and learn how evidence-based practices can be put into action through protocols and collaborative approaches that can then be practiced using simulation scenarios.

Human Patient Simulation Network (HPSN) Conferences
HPSN World 2020: More Info Coming Soon!
OB Care & Management Workshop: March 2-3 at Jacksonville University Healthcare
HPSN Regional SimDay Event: Fayetteville, Arkansas - April 7th POSTPONED
CAE's largest Human Patient Simulation Network conference is back! In 2020 they will be looking for you to join them around the world and in Orlando and share your passion for healthcare education through simulation. No matter your background, no matter your experience, HPSN World never fails to inspire. Choose from over 100 sessions on simulation-based learning and earn CEUs. Enjoy the stimulating keynote presentations and interactive special events. Select from a range of pre-conference training courses. Be the first to experience exciting new methodologies and technologies. Collaborate and connect with a truly global medical simulation community, and more!

iSIM Courses
(Various Dates Throughout 2020)
By UPMC WISER, UM GCRME, and UH SimTiki
These 3-day internationally renowned workshop programs, created in collaborative effort between WISER at the University of Pittsburgh and the Gordon Center for Research in Medical Education at the University of Miami, is designed as an introduction to fundamental skills and abilities for delivering simulation-based healthcare education through a variety of techniques and technologies. The program emphasizes hands-on activities and active participation to maximize simulation-based instruction skill acquisition. Class group sizes are kept small to allow for maximum participation. The primary audience for this course are healthcare educators wishing to improve their skills as instructors in simulation education.
 International Society for Quality in Health Care
International Society for Quality in Health Care
CANCELLED August 30 - September 2nd, in Florence Italy (Returning 2021)
ISQua conferences provide a platform for like-minded peers to network, share their ideas and foster innovation all in the hope to improve and support safe health care worldwide ISQua's annual international conference is taking place from 30th August to 2nd September 2020. Join over 1,500 medical and health care professionals from over 80 countries for our quality in health care conference 2020. The theme of this year's conference is 'Emotion, Inspiration and Creativity: Pathways to Global Health Quality'. This year's health care convention will host over 180 expert presentations and provide ample opportunity for networking among the best and brightest in the international health care quality community.
 SimONE SIM Expo
SimONE SIM Expo
Toronto, Canada - October 19th - 20th
Join Canada's passionate, interprofessional healthcare simulation community and world-leading experts at the 2020 SIM Expo! SIM Expo continues to be the largest interprofessional and multidisciplinary simulation conference in Canada and they are excited to bring it to Toronto for 2020! Each year, the SIM Expo brings together healthcare leaders in university, college, hospital, emergency services, and community care sectors. Their participants are deans, administrators, educators, clinicians, SIM centre managers and directors, and researchers from all health professions. Most participants are Canadian but it is an open conference and each year they are honored to host some international participants.

New Zealand Association for Simulation in Healthcare 2020 Conference
CANCELLED November 6-7 in Manawa, Christchurch
On behalf of the Canterbury Collaborative Simulation Interest Group (CCSIG), it is with great excitement and pleasure that we invite you to join us for the New Zealand Association for Simulation in Healthcare (NZASH) Conference 2020. The conference theme this year is “Connect and Collaborate”. Although we use simulation to develop better teamwork strategies, are we really connecting with everyone to ensure a positive journey for our patients? When times get tough the Midwife need the Paramedics and Medical staff, the Emergency team need the input from Paediatricians, ICU and Allied Health staff. In 2020, NZASH would like to challenge you to invite and work with a department/speciality you have not considered as part of your team, you will be amazes how much you learn. The programme has been designed with Connection and Collaboration at its centre with workshops, presentations and simulations being designed, delivered and debriefed by a variety of different professions. Demonstrating that simulation whatever the scenario can reach many specialities.
 Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference
Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference
November 9th - 11th in Birmingham, England
ASPiH is a not-for-profit membership Association comprising of members drawn from healthcare, education and patient safety backgrounds including researchers, learning technologists, workforce development or education managers, administrators, and healthcare staff and students. All have a genuine interest in new and innovative methods of learning, as well as optimizing the use of existing simulation resources within their practice. Their membership bridges undergraduate and pre- registration education as well as postgraduate and post registration training and on-going CPD for all of the health and social care workforce. This annual event is the premiere UK-based event for all things clinical simulation!
 International Pediatric Society for Simulation (IPSS) Symposium
International Pediatric Society for Simulation (IPSS) Symposium
POSTPONED to October 23-26, 2020 April 26-29 in St. Petersburg, FloridaRenowned global experts will gather for the world’s largest meeting dedicated exclusively to pediatric and perinatal simulation. Over three days, attendees will discuss the role simulation plays to provide safe and effective care to sick children and infants, and the continued evolution and expansion of pediatric simulation across the globe. Experience hands-on and immersive simulations at one of the country’s leading centers during pre-conference and afternoon workshops. Learn from the experts and see the highest quality simulators in the market. Learn the latest advancements in pediatric and neonatal simulation. Expand your horizons through the large variety of education sessions in various tracks offered at IPSSW2020. Connect with industry professionals from all over the globe and see the products, services and the latest technology in pediatric simulation!
IPSS Virtual Meeting: Tuesday, April 28 2:00 pm – 3:30 pm EDT
Leaders in pediatric simulation will discuss using simulation as a tool for discovering new processes and procedures during a pandemic. Panelists:
Tensing Maa, MD | Nationwide Children’s Hospital
Jennifer Reid, MD | Seattle Children’s
Stephanie Sudikoff, MD | Yale New Haven Hospital
2020 Medical Simulation Conferences
General Conference Listings:
- International Meeting for Simulation in Healthcare - January 18-22 in San Diego, California
- Operational Medicine Symposium - January 22-23 at Parma Payne Goodall Alumni Center in San Diego, CA
- 5th Pediatric Simulation Training and Research Society of India (PediSTARS) Conference - February 6-11 in Chennai, India
- Virtual Reality and Healthcare Symposium - March 2-3 in Nashville, Tennessee
- European Society of Anaesthesiology Patient safety Policy Summit - March 3-4 in Brussels
- CANCELLED Patient Safety Movement Foundation's Annual World Patient Safety, Science & Technology Summit - March 5-7 in Huntington Beach, California
- CANCELLED Dutch Society for Simulation in Healthcare (DSSH) Congress - March 18 in Maastricht, Netherlands
- POSTPONED George Washington University Healthcare Simulation Conference 2020 - March 19-20 in Ashburn, VA
- CANCELLED Virtual Medicine at Cedars-Sinai - March 25-26 in Los Angeles, California
- POSTPONED 8º Congreso Nacional Sociedad Española de Simulación Clínica y Seguridad del Paciente - 26-28 Marzo in Granada Spain
- POSTPONED SimGHOSTS-X One Day Event - April 3rd, UTHSC Memphis, Tennessee
- POSTPONED FDA Public Workshop on Simulation Practices in Health - April 13-15 in Silver Springs, MD
- POSTPONED Simulation in Rural Healthcare Conference (SIRH) - April 22-23 in Sioux Falls, South Dakota
- POSTPONED International Pediatric Society for Simulation (IPSS) Symposium - April 26-29 in St. Petersburg, Florida
- CANCELLED MODSIM World - May 5-7 in Norfolk Virginia
- CANCELLED IHI Patient Safety Congress - May 13-15 in Orlando, Florida
- POSTPONED AvKin's University of Delaware Simulated Participant Conference - May 14-15 in Newark, Delaware
- CANCELLED 5th Biennial 2020 National League for Nursing/Boise State Simulation Conference - May 14 - 16 in Boise, Idaho
- CANCELLED National Forum on Simulation for Quality & Safety - May 29th in Halifax, Canada
- Scottish Healthcare Skills Network Conference - June 9-10 in Inverness, Scotland
- POSTPONED Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) - June 13-17 in Phoenix Arizona
- POSTPONED Society in Europe for Simulation Applied to Medicine (SESAM) Annual Conference - June 17-19 in Milan, Italy
- Converted to Virtual Event! Serious Play Conference - June 23-25, Online
- CANCELLED CALSIM 2020 Inaugural Simulation Conference - June 27-28 at Cedars Sinai in Los Angeles, CA
- Converted to Virtual Event! International Nursing Association for Clinical Simulation & Learning (INACSL) Conference - June 24-27, Online
- Converted to Virtual Event! Academy of Communication in Healthcare Virtual Research Forum - June 26-27, Online
- CANCELLED Pacific Northwest Healthcare Simulation Collaborative Conference - July 7-9 in Seattle, Washington
- CANCELLED UK Patient Safety Congress – July 13-14 in Manchester Central, United Kingdom
- CANCELLED Virginia State Simulation Alliance (VASSA) Conference - August 3-5 in Williamsburg, VA
- Converted to Virtual Event! SimGHOSTS 2020 Annual Global Symposium - August 4-7, Online
- Converted to Virtual Event! Association for Standardized Patient Educators Annual Conference (ASPE) - August 12-14, Online
- CANCELLED International Society for Quality in Health Care (ISQua) - August 30 - September 2nd, in Florence Italy
- CANCELLED SimONE SIM Expo - October 19th - 20th in Toronto, Canada
- CANCELLED New Zealand Association for Simulation in Healthcare 2020 Conference - November 6-7 in Manawa, Christchurch
- Converted to Virtual Event! Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference - November 9th - 11th, Online
- Virtual HPSN Regional SIMDAY West Coast by CAE Healthcare - November 18th - 19th, Online
- Converted to Virtual Event! Laerdal Simulation User Network (SUN) Regional Conferences - Various Dates & Locations
- Converted to Virtual Event! SIMex 2020: Fourth International Congress on Clinical Simulation - November 18th - 20th, Online
The following sim conferences are biennial and were set return in 2021:
However, because of COVID-19 they may still be affected!
- Congreso Federación Latinoamericana de Simulación Clínica y Seguridad del Paciente
- STARS: Simulation and Training for Resilience and Safety (Multiple Industries)
- SESAM/SimGHOSTS/SingHealth S3 Singapore Conference
- Australasian Simulation Congress (Multiple Industries)
- Tennessee Simulation Alliance Conference - Dates & Location TBA
General Workshop Listings:
- WISER Simulation Center Courses (Administration, Sim Tech Ops, Debriefing, Center Design & More) - Various Dates Throughout the Year
- iSIM Debriefing Course from WISER, SimTiki, and GCRME - March 30-31, May 11-12, September 14-15, November 2-3
- Military Moulage Workshops - Various Dates and Locations
- Moulage Concepts Workshops Around the United States - Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) - Various Dates and Locations
- Simulation Professionals of Texas (SPOT) Connections & Collaborations - Various Dates
- The Debriefing Academy Master Debriefer Course - Various Dates & Locations
- George Washington University Simulation Workshops – Various Dates in Washington D.C.
- Jump Simulation Courses: Instructor, Debriefer and More - Various Dates
- Boston Children's Hospital: Simulation Courses - Various Dates in Boston, MA
- California Simulation Alliance Education Courses - Various Dates around California
- University of Illinois Professional Development Programs for Standardized Patient Educators - Various Dates in Chicago, Illinois
- Colloque simulation en santé - June 10-11 in Joliette, Canada
- Maryland Community & College Simulation Users Network – June 2-3 in Maryland
- RUSH Simulation and Procedural Bootcamp for Emergency Medical Clinicians – March 18-20 and May 18-20 in Chicago, Illinois
- Level 3 Healthcare Sim Tech Bootcamp
April 14-16th, 2020POSTPONED TO: SEPTEMBER 15TH-17TH,
Don't see your listing or want to be featured? Email us the event title, dates, location, the link and that you would like to be featured!
Concluded 2020 Featured Events:
 Operational Medicine Symposium
Operational Medicine Symposium
January 22-23, 2020
Parma Payne Goodall Alumni Center in San Diego, CA
The 2nd Annual Operational Medicine Symposium will focus on DoD initiatives to enhance military medicine techniques and improve the quality of care of our nation’s warfighters. This symposium will bring together highly regarded medical professions from all services to discuss the practice of medicine in expeditionary environments from battlefield to installation. Topics to be Covered at the Symposium: Examining How the Changing Operating Environment Will Impact Operational Medicine, Update on the Realignment of the Military Health System and the Transition of DHA’s New Responsibilities,Ensuring Military Medical Forces are Ready and Equipped to Care for the Warfighter in Expeditionary Environments, and more!
 VR Voice Healthcare Conference
VR Voice Healthcare Conference
(Use discount code: healthysim)
March 2-3 at Vanderbilt University, Nashville TN
Now in its 4th year, the VR and Healthcare Global Symposium presents a balanced mix of speakers from academia, healthcare organizations and the virtual reality technology industry. This year’s annual event takes place next month at the Vanderbilt University on March 2nd and 3rd in Nashville, TN. With over 60 speakers will present over 2 days in both a plenary and track breakout format, this is the largest conference in the world dedicated to the development of virtual reality in healthcare. And great news, HealthySimulation.com has partnered with the VR Voice team to offer you 30% off your event registration with discount code "healthysim"!
 Annual World Patient Safety, Science & Technology Summits
Annual World Patient Safety, Science & Technology Summits
CANCELLED March 5-7, 2020
Huntington Beach, California
The 8th Annual World Patient Safety, Science & Technology Summit is co-convened with the International Society for Quality in Health Care (ISQua) and American Society of Anesthesiologists (ASA), and the European Society of Anesthesiology (ESA) and will bring together all stakeholder groups to discuss novel solutions to the leading challenges facing hospitals today. We invite international hospital leaders, medical and information technology companies, the patient advocacy community, public policy makers and government officials to join us for our 8th Annual event in 2020 and be part of this momentous event as we share our plans post-2020. The Summit featured keynote addresses from public figures, patient safety experts and plenary sessions with healthcare luminaries, and patient advocates, as well as announcements from organizations who have made their own commitments to reach the Patient Safety Movement Foundation’s goal of ZERO preventable deaths by 2020.
Featured Events:

Patient Safety Movement Foundation Mid Year Planning Meeting
September 17, 2019 (World Patient Safety Day!) at UC Irvine Health, CA
The Patient Safety Movement Foundation’s Midyear Planning Meeting, co-convened with UC Irvine Health, will take place on Tuesday, September 17th, 2019, at the Beckman Center of the National Academies of Sciences & Engineering, University of California, Irvine (UCI) on World Patient Safety Day! This full-day working meeting will bring together one hundred global stakeholders across healthcare for an intimate examination and discussion about how we can continue planning for ZERO preventable deaths in hospitals. This meeting allows an opportunity for participants to provide their experiences and expertise as we propel our mission of ZERO forward.
Annual World Patient Safety, Science & Technology Summits
January, 2020
Huntington Beach, California
The 7th Annual World Patient Safety, Science & Technology Summit will be co-convened by the American Society of Anesthesiologists and the European Society of Anaesthesiology. The 2019 Summit will gather international hospital leaders, medical and information technology companies, the patient advocacy community, public policy makers and government officials, to discuss solutions to the leading challenges that cause preventable patient deaths in hospitals worldwide.
International Society for Quality in Health Care (ISQua) Patient Safety Conference
October 20th-23rd in Cape Town, South Africa
The International Society for Quality in Healthcare Care, or ISQua, is a member-based, not-for-profit community and organization dedicated to promoting quality improvement in health care, who have been working to improve the quality and safety of health care worldwide for over 30 years! Their extensive network of health care professionals spans over 70 countries and 6 continents all of whom share the goal of improving patient safety through education, knowledge sharing, external evaluation, supporting health systems worldwide and connecting like-minded people through their health care networks.
And additionally....

SimGHOSTS Hands-on Simulation Technology Conferences
SimGHOSTS Annual Hands-On Simulation Technology Operations Symposiums
August 4-7th, 2020
Baylor University
Dallas, TX
See Website for 2020 Information.
SimGHOSTS 2019 “S3” Singapore Event
In collaboration with SESAM and SingHealth
October 22nd – 25th, 2019
SingHealth, Singapore
Additional International Events TBA, See Website for Details!
 International Nursing Association for Clinical Simulation and Learning (INACSL) Conference 2020
International Nursing Association for Clinical Simulation and Learning (INACSL) Conference 2020
June 24-27, 2020
Raleigh, NC
Conference Location: Raleigh Convention Center

Human Patient Simulation Network (HPSN) Conferences
World Event Feb. 26-28 (2019) in Orlando, Florida
*Update* This event will be live streamed so stay tuned for details! CAE's largest Human Patient Simulation Network conference is back! In 2019 they will be looking for you to join them in the UK and Orlando and share your passion for healthcare education through simulation. No matter your background, no matter your experience, HPSN World never fails to inspire. Choose from over 100 sessions on simulation-based learning and earn CEUs. Enjoy the stimulating keynote presentations and interactive special events. Select from a range of pre-conference training courses. Be the first to experience exciting new methodologies and technologies. Collaborate and connect with a truly global medical simulation community, and more!

iSIM Courses
October 9 - 11 in Pittsburgh, Pennsylvania (More Dates Below)
By UPMC WISER, UM GCRME, and UH SimTiki
This 3-day internationally renowned program, created in collaborative effort between WISER at the University of Pittsburgh and the Gordon Center for Research in Medical Education at the University of Miami, is designed as an introduction to fundamental skills and abilities for delivering simulation-based healthcare education through a variety of techniques and technologies. The program emphasizes hands-on activities and active participation to maximize simulation-based instruction skill acquisition. Class group sizes are kept small to allow for maximum participation. The primary audience for this course are healthcare educators wishing to improve their skills as instructors in simulation education.
SimONE SIM Expo:
October 21-22 in Montreal, Canada
Join Canada's passionate, interprofessional healthcare simulation community and world-leading experts at the 2019 SIM Expo! SIM Expo continues to be the largest interprofessional and multidisciplinary simulation conference in Canada and they are excited to bring it to Montréal for 2019! Each year, the SIM Expo brings together healthcare leaders in university, college, hospital, emergency services, and community care sectors. Their participants are deans, administrators, educators, clinicians, SIM centre managers and directors, and researchers from all health professions. Most participants are Canadian but it is an open conference and each year they are honored to host some international participants.
Congreso Federación Latinoamericana de Simulación Clínica y Seguridad del Paciente: October 30th - November 1st in Cancun, Mexico
In 2007 the Latin American Society for Simulation in Healthcare (ALASIC) was founded by members from different Northern, Central and South American countries with the idea of building collaboration and networking from across the continent. In 2017, the FLASIC organization was born from ALASIC to act a federation of smaller regional simulation societies, which now maintains almost 1,000 members from 7 different societies who can collaborate and share via an online network. Every 2 years FLASIC holds its biennial conference with the 2019 being held in Cancun this October 30th to November 1st. The event will host workshops, lectures, posters, and an exhibit hall, all in an all inclusive hotel right near the beach! Keynote speakers include José Maestre from Spain, speaking on simulation faculty development, Marlova Silva from Chile speaking on simulation implementation, Roger Daglius from Brazil speaking on clinical simulation innovation, and Grl. Dr. Octavio Ruíz from Mexico speaking on leadership in healthcare simulation.
Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference: Nov. 4 - 6 in Belfast, Ireland
ASPiH is a not-for-profit membership Association comprising of members drawn from healthcare, education and patient safety backgrounds including researchers, learning technologists, workforce development or education managers, administrators, and healthcare staff and students. All have a genuine interest in new and innovative methods of learning, as well as optimizing the use of existing simulation resources within their practice. Their membership bridges undergraduate and pre- registration education as well as postgraduate and post registration training and on-going CPD for all of the health and social care workforce. This annual event is the premiere UK-based event for all things clinical simulation!
2019 Medical Simulation Conferences
General Conference Listings:
- NLN/University of Central Florida Simulation Conference – March 7 - 8 in Orlando, Florida
- Dutch Society for Simulation in Healthcare (DSSH) Congress – March 13 in Rotterdam, Netherlands
- Virtual Reality and Healthcare Symposium – March 7 - 8 in Tucson, Arizona
- NLN/University of Central Florida Simulation Conference – March 7-8 in Orlando, FL
- Virtual Medicine 2019 at Cedars-Sinai – March 27 - 28 in Los Angeles, California
- Tennessee Simulation Alliance Conference – March 27 - 28 at University of Tennessee Health Science Center in Knoxville, Tennessee
- STARS: Simulation and Training for Resilience and Safety (Multiple Industries) – March 26 – 27 in London, UK
- 7º Congreso Nacional Sociedad Española de Simulación Clínica y Seguridad del Paciente - 4 al 6 de Abril. Campus de Villaviciosa de Odón (Spain)
- IHI Summit of Improving Patient Care – April 11 -13 in San Francisco, CA
- Simulation in Rural Healthcare Conference (SIRH) – April 16 - 18 in Omaha, Nebraska
- NYC Health + Hospitals Simulation Center 3rd Symposium - April 23 in the Bronx, NY
- International Pediatric Society for Simulation (IPSS) Symposium – May 20 - 22 in Toronto, Canada
- National Forum on Simulation for Quality & Safety – May 28 in Vancouver, Canada
- Association of Standardized Patient Educators Annual Conference (ASPE)– June 8 - 11 in Orlando Florida
- Society in Europe for Simulation Applied to Medicine (SESAM) Annual Conference – June 12 - 14 in Glasgow, UK
- International Nursing Association for Clinical Simulation & Learning (INACSL) Conference – June 19 - 22 in Phoenix, Arizona
- UK Patient Safety Congress – July 2-3 in Manchester Central, UK
- University of Cincinnati College of Nursing 7th iCoN – July 9-11 in Cincinnati, OH
- Simulation Professionals of Texas Connections & Collaborations – August 9th, 10AM-2PM at Texas Christian University in Fort Worth, TX
- Australasian Simulation Congress (Multiple Industries) – September 2 - 5 in Gold Coast, Australia
- Spectrum Health Simulation Conference 2019: Discover the Possibilities – September 9th at Grand Valley State University in Grand Rapids, MI
- Kansas City Healthcare Simulation Conference – September 20th at Johnson County Community College in Overland Park, KS
- Columbia University SON Innovations in Simulation Summit – October 11th at Columbia University School of Nursing in NY, NY
- Arizona Simulation Network 9th Annual Conference – October 12th at Grand Canyon University in Phoenix, AZ
- SimONE SIM Expo – October 21-22 in Montreal, Canada
- AlaSim Conference – October 24th in Huntsville, Alabama
- International Society for Quality in Health Care (ISQua) Patient Safety Conference – October 20th-23rd in Cape Town, South Africa
- Congreso Federación Latinoamericana de Simulación Clínica y Seguridad del Paciente – October 30th - November 1st in Cancun, Mexico
- Association for Simulated Practice in Healthcare (ASPiH UK) Annual Conference – November 4 - 6 in Belfast, Ireland
- Sim Summit Presented by the Royal College of Physicians and Surgeons of Canada – November 7 - 8 in Winnipeg, Canada
- Turkish Association for Medical Education Simulation Conference – November 13 - 14 in Istanbul, Turkey
- Clinical Simulation Educator Programme at Pakistan's The Aga Khan University – December 9 - 13 in Karachi , Pakistan
- Laerdal Simulation User Network (SUN) Regional Conferences - Various Dates
General Workshop Listings:
- WISER Simulation Center Courses (Administration, Sim Tech Ops, Debriefing, Center Design & More) - Various Dates Throughout the Year
- Moulage Concepts Workshops Around the United States - Various Dates
- Mayo Clinic Instructor Courses (Debriefing Basics, Comprehensive, Development) - Various Dates and Locations
- UK-Based WISER WATFORD Regional Simulation Event - March 12 in Watford, UK
- Northern Ontario Healthcare Simulation Symposium – May 8th in North Bay, Canada
- 2019 OHCWC Simulation Conference – May 21, 2019 at Oklahoma State University in Oklahoma City
- Colloque simulation en santé – May 30 - 31 in Joliette, Canada
- The Debriefing Academy Master Debriefer Course – May 30 to June 2 in Calgary, Canada
- Fundamentals of Debriefing – May 29 - June 5 & July 29 - August 6 in Albuquerque, New Mexico
- Maryland Community & College Simulation Users Network – June 4th - Hosted By Community College of Baltimore County Catonsville Campus
- George Washington University Simulation Workshops – Various Dates in Washington D.C.
- iSIM Debriefing Course from WISER & GCRME – July 29 - 31 in Pittsburgh, Pennsylvania
- iSIM Debriefing Course from WISER & GCRME – October 9 - 11 in Pittsburgh, Pennsylvania
- RUSH Simulation and Procedural Bootcamp for Emergency Medical Clinicians – October 14 - 16 in Chicago, IL
- Jump Simulation Courses - Various Dates
- Level 3 Healthcare Sim Tech Bootcamp April 14-16th, 2020
- Intentional Simulation Center Management: Tactics for Sustainability Conference
- Boston Children's Hospital: Foundations of Simulation Instruction Workshop - November 19 21 in Boston, MA
2018 Healthcare Simulation Conferences (Featured)

Alabama Simulation (AlaSim) International Conference
May 22-23
Huntsville, Alabama
The annual AlaSim International Conference & Exposition showcases the vibrant, multi-domain, modeling and simulation (M&S) industry in Alabama. The event takes place May 22-23, 2018 at the Holiday Inn Huntsville in Huntsville, Alabama. Abstracts submission will remain open until March 18th. The conference is designed to focus on both the breadth and depth of simulation activity in Alabama and to collect, document, display, and discuss the current state of simulation technology throughout the world.
National Modeling & Simulation Coalition (NM&SC) 2018 Meeting
Sept. 25- 26 at UNMC, Omaha NE
Registration is now open for the National Modeling & Simulation Coalition (NM&SC) 2018 National Meeting, which will be held Sept. 25- 26 at UNMC. Co-hosted by iEXCELSM and the National Strategic Research Institute (NSRI), the focus of the interdisciplinary meeting is on “Improving Human Performance and Effectiveness” and will feature world-class keynote speakers and highlight four engaging panel sessions led by subject matter experts in the fields of simulation and visualization. Keynote speakers are Jeffrey P. Gold, M.D., chancellor, UNO & UNMC; Daniel Serfaty, president/CEO, Aptima, Inc.; and Robert Amyot, M.D., president, CAE Healthcare.
SIM-One Forum on Simulation in Quality June 12th, &
2018 SIM Expo – Nov 13-14 in Calgary, AB
Save the date for SIM-One's 3rd National Forum which will be held on June 12, 2018, in Toronto at the George Brown College Waterfront Campus looking over Lake Ontario. Expect an incredible lineup of speakers and presentations on leading-edge applications of simulation to advance patient safety and quality improvement efforts. Not yet announced is the annual SIM Expo event, which this year will take place in Calgary. Each year, the SIM Expo brings together healthcare leaders in university, college and hospital sectors, comprised of administrators, educators, clinicians, SIM centre managers and directors, and researchers from all health professions across Canada and beyond. The SIM Expo prides in its strong culture of networking, building relationships, and uniting the simulation community. The annual conference allows participants to connect directly with peers, experts and future collaborators in healthcare simulation.

Human Patient Simulation Network (HPSN) Conferences
UK June 20th-21st in Nottingham &
World Event Feb. 26-28 (2019) in Orlando, Florida
CAE's largest Human Patient Simulation Network conference is back! In 2019 they will be looking for you to join them in the UK and Orlando and share your passion for healthcare education through simulation. No matter your background, no matter your experience, HPSN World never fails to inspire. Choose from over 100 sessions on simulation-based learning and earn CEUs. Enjoy the stimulating keynote presentations and interactive special events. Select from a range of pre-conference training courses. Be the first to experience exciting new methodologies and technologies. Collaborate and connect with a truly global medical simulation community, and more!

SimGHOSTS Hands-on Simulation Technology Conferences
SimGHOSTS / Simulation Australasia / ASSH 4th Hands-on Simulation Technology & Clinical Learning Event
June 27th-29th
Sunshine Coast, Australia
SimGHOSTS 7th Hands-On Simulation Technology Operations Symposium
July 31st-Aug.3rd
Memphis, Tennessee
Join hundreds of Simulation Champions from around the world at these annual international hands-on training events! The “Gathering of Healthcare Simulation Technology Specialists” which will be operating its eighth year as a US-based non-profit 501(c)3 organization based out of Las Vegas, Nevada. The 2018 SimGHOSTS events will provide a meeting place for you to exchange ideas and network with peers as well as receive specialized training in: Manikin hardware repair and software programming, Audiovisual equipment debugging, IT networking and infrastructures, Moulage makeup, Team communication & leadership techniques, Research methodology, Serious Games, VR and AR and much more.

New Zealand Association of Simulation in Healthcare Conference 2018
Waitemata Simulation Centre, Awhina Health Campus, Waitakere Hospital, West Auckland
Friday 26 & Saturday 27 October 2018
The NZASH 2018 Conference is a great opportunity to share knowledge and build relationships within the simulation community. If you are wanting to learn more about simulation, see what others are doing, share your knowledge and expertise, or just want to see if simulation is right for you, we welcome you to join us for what will be a fantastic program of presentations, discussion and workshops! Their conference theme is ‘Patient First’, reflecting the need and ability of simulation in healthcare to contribute to patient centered care and to place patient safety at the core of everything we do. In 2018 they will also take up the challenge of exploring patient involvement in planning, designing and delivery of training. The format of the program will be held over two days, with concurrent sessions and workshops encouraging the engagement of delegates.
2018 Medical Simulation Conferences (General)
- Dutch Society for Simulation in Healthcare (DSSH) 10th Congress - March 14th in Enschede, The Netherlands
- Simulation in Rural Healthcare Conference - March 20th-22nd in Bozeman, Montana
- Saudi Health Simulation Conference - April 23-25 at Four Seasons, Riyadh Saudi Arabia
- International Pediatric Society for Simulation (IPSS) 10th Symposium - May 14th-16th in Amsterdam, The Netherlands
- 4th Boise State University & NLN Biennial Simulation Conference - May 17-19, Boise, Idaho
- International Nursing Association for Clinical Simulation & Learning (INACSL) 17th Conference - June 13th-16th, Toronto, Canada
- Association of Standardized Patient Educators Annual Conference - June 17th-20th, Kansas City, Missouri
- Society in Europe for Simulation Applied to Medicine (SESAM) 24th Annual Conference - June 27th-29th, Bilbao, Spain
- Royal College of Physicians of Canada's Simulation Summit - September 28th+29th, Ottawa, Canada
- Association for Simulated Practice in Healthcare 2018 Event - Nov.13th-15th, Southport UK
Also, don't forget to check out healthcare simulation conferences from leading vendors including:
- Laerdal Simulation User Network (SUN) Conferences - Apr. 30th-May 2nd, Schaumburg, Illinois and October 9-11 in Orlando, Florida
Email us to have your healthcare simulation conference listed.
Sponsored Content: